Autologous platelet-rich fibrin matrix in the treatment of diabetic foot ulcers: A case series
Received:
Accepted:
Published:
Authors: Darwin Britto D, Rajesh Kesavan, Changam Sheela Sasikumar, Aarthi V S, Ajithkumar M, Anya G and Gokulnath R
Citation:
Britto D, Kesavan R, Sasikumar CS et al (2025) Autologous platelet-rich fibrin matrix
in the treatment of diabetic foot ulcers: A case series. Global Wound Care Journal 1(1): 36-41
Declarations:
None.
Financial support
Nil.
Conflict of interest:
The authors have no conflict of interest to disclose.
Acknowledgements:
The authors thank the research team and Outpatient staff of Dr RK Diabetic Foot Care and Podiatric Institute and Rakesh Jhunjhunwala Amputation Prevention Centre for their assistance and valuable input.
Correspondence:
Dr Rajesh Kesavan, Dr RK Diabetic Foot and Podiatry Institute, No 1 Jayanthi Nagar Extension, off 200 Feet Rd, Kolathur, Chennai 600 099, India. E: drrkdiabeticfoot@gmail.com
DOI: https://doi.org/10.63896/gwcj.1.1.36
Diabetic foot ulcers remain a significant challenge in clinical practice, necessitating innovative strategies to improve healing outcomes. This case series investigates the clinical impact of autologous platelet-rich fibrin matrix (PRFM) in the treatment of non-healing diabetic foot ulcers. By meticulously examining five cases, the study reveals compelling outcomes, including substantial reductions in ulcer size and accelerated healing. Patient-specific considerations, along with a focus on adverse events, provide a comprehensive understanding of the impact of PRFM. The case series underscores the promising role of PRFM as a game-changing adjunctive therapy for challenging diabetic foot ulcers, urging further exploration and integration into mainstream clinical practice.
Diabetic foot ulcers (DFUs) are a persistent and challenging complication of diabetes, often characterised by impaired wound healing and an increased risk of infection (Kale et al, 2023). The intricate interplay of factors such as compromised vascularity, neuropathy, and immune dysfunction contribute to the delayed healing observed in these wounds. Managing DFUs is a challenge due to the high recurrence rate. Within 1 year of healing, around 40% of patients experience a recurrence. This increases to 60% within 3 years and 65% within 5 years (Fibrini et al, 2022). Conventional wound care approaches have limitations in promoting timely and effective healing in DFUs, necessitating the exploration of novel therapeutic strategies.
Autologous platelet-rich fibrin matrix (PRFM) has emerged as a promising adjunct in the wound healing armamentarium. PRFM is rich in platelets and growth factors that play pivotal roles in orchestrating various stages of the wound healing process (Nagaraju et al, 2017). The application of PRFM aims to harness these regenerative properties to accelerate healing, reduce inflammation and mitigate complications associated with DFUs. The use of autologous PRFM has shown promise in expediting the wound healing process by promoting tissue regeneration and angiogenesis (Xu et al, 2020).
This series of five cases aims to contribute to the growing body of evidence supporting the effectiveness of PRFM therapy in the management of DFUs.
This study was approved by the Institutional Ethics Committee of Hycare Super Speciality Hospital (Project No:50/HSSH-EC/2023). All subjects provided written informed consent for publication.
Preparation and application of PRFM
To prepare the PRFM, 10ml of blood is drawn from the patient using a flash needle and infused into a vacutainer tube. The tube and a balancing tube are centrifuged at 4,000 rpm for 10 minutes in either a Remi R 303 or Remi 4c/8c centrifuge (Remi, Mumbai, Maharashtra, India) [Figure 1].
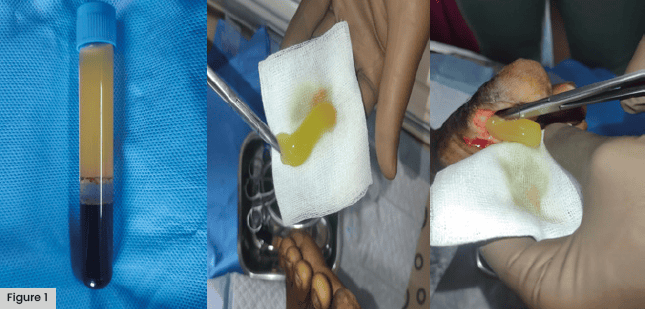
To apply PRFM, the wound is then cleaned using normal saline. The prepared PRFM matrix is applied to the wound and gently stuffed into the cavity area. Any remaining platelet-poor plasma is poured onto the wound surface. The wound is covered with Mepitel dressing (Molnlycke Health Care, Mikkeli, Finland), overlaid with sterile gauze, and wrapped with a non-woven dressing.
The wound is left covered for 4–5 days. At dressing changes, the wound is cleaned with normal saline and a Mepitel dressing applied. This method outlines a systematic approach to harnessing autologous PRFM for wound healing, emphasising meticulous preparation, application, and post-application care.
Case presentations
Case 1
A 67-year-old woman with type 2 diabetes (T2D) of 27 years’ duration, with a 10-year history of systemic hypertension and recently diagnosed peripheral vascular disease had undergone a left trans-metatarsal amputation due to complications associated with diabetes 9 years earlier. She presented with a necrotic ulcer on the stump of Wagner grade 2.
The patient underwent wound debridement, followed by a left superficial femoral artery/popliteal and peroneal angioplasty. Post-operatively, she received IV antibiotic therapy for 5 days, consisting of 2 g ceftriaxone once daily (Zydus Healthcare, India) and 600 mg clindamycin three times daily (Zen Pharma, India). Lab results after angioplasty showed HbA1c 64 mmol/l, haemoglobin (Hb) 6.27 mmol/l, platelets 280 × 109/l, white blood cells (WBC) 6.3 × 109/l, serum creatinine 159.1 µmol/l and C-reactive protein (CRP) 490 mg/l.
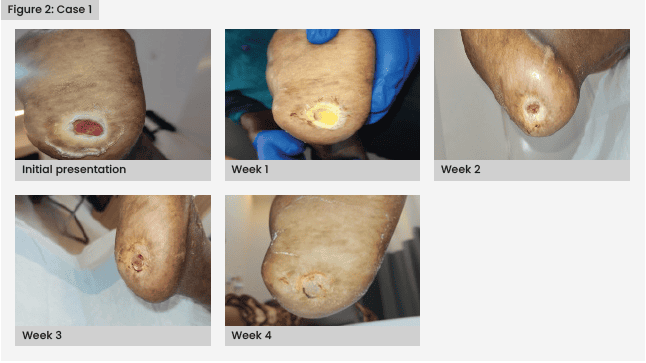
Once the wound showed no signs of infection, with granulation formation, the patient was considered for PRFM therapy to expedite wound healing. Regular monitoring revealed a significant improvement in wound healing by the third week, marked by a reduction in size. Continuation of PRFM therapy resulted in complete wound healing by the fifth week [Figure 2].
Case 2
This case was an 84-year-old man with T2D (20 years), systemic hypertension (20 years), hypothyroidism (3 years), chronic kidney disease (8 months) and coronary artery disease (16 years), and a cerebrovascular accident 10 years earlier. His toe had been amputated recently. The patient presented with an infected non-healing DFU of grade 2 on the left foot third toe amputation stump.
Surgical intervention involved the excision of the third metatarsal head and debridement, followed by antibiotic beads placement. IV antibiotics were administered for 5 days, consisting of clindamycin 600 mg three times daily (Zen Pharma, India) and 1.5 g cefoperazone with sulbactam twice daily (Aqua Vitoe Laboratories, India).
Lab results after surgery were HbA1c 93 mmol/mol, Hb 5.65 mmol/l, platelet count of 134 × 109/1, WBC 12.47 × 109/1, serum creatinine 247.5 µmol/l and CRP 230 mg/l.
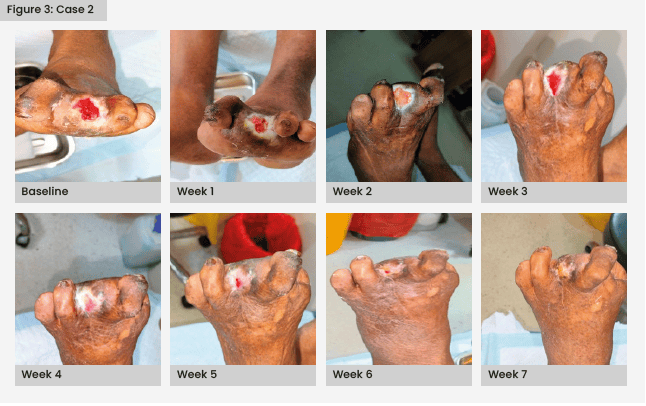
Following successful infection control, autologous PRFM therapy was initiated. By the fifth week, a significant improvement in wound healing was observed and complete healing was achieved by the seventh week [Figure 3].
Case 3
An 83-year-old woman with systemic hypertension for 20 years and recently diagnosed peripheral vascular disease presented with an infected ulcer on the right foot great toe, with septic arthritis of the interphalangeal joint of Wagner grade 2.
The patient underwent right great toe bone curettage with debridement, followed by right superficial femoral artery plasty and stenting, and popliteal/tibial plasty. IV antibiotics, were administered for 5 days – clindamycin 600 mg three times daily (Zen Pharma, India) and ceftriaxone 2 g once daily (Zydus Healthcare, India).
At the time of debridement, the lab results were random blood sugar 6.8 mmol/l, serum creatinine 97.2 µmol/l, platelet count 250 × 109/1, WBC 11.6 × 109/1, Hb 6.76 mmol/l, and CRP 650 mg/l.
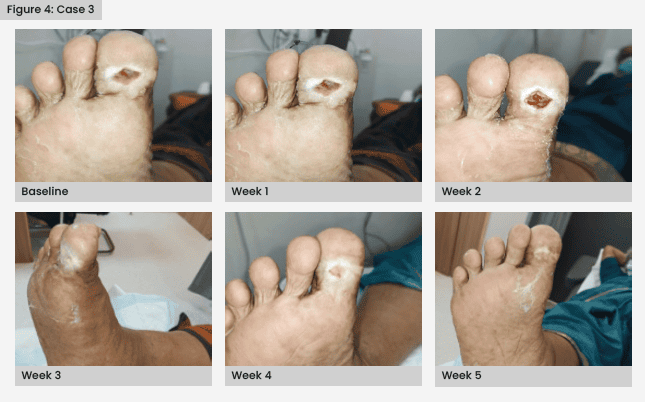
Once there were no signs of wound infection, the patient underwent autologous PRFM therapy. By the third week, there was a significant improvement in wound healing, and complete healing was achieved by the fifth week [Figure 4].
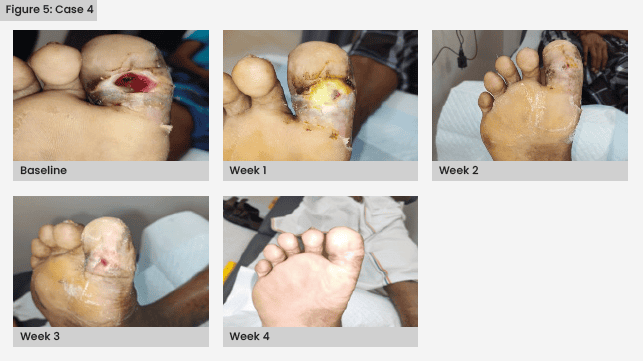
Case 4
A 67-year-old man with T2D presented with an infected ulcer on the right foot great toe, with septic arthritis of the right great toe interphalangeal joint of Wagner grade 2.
The patient underwent a right great toe bone curettage with debridement. IV antibiotics were administered for 5 days, consisting of clindamycin 600 mg three times daily (Zen Pharma, India) and 3 g cefoperazone with sulbactam twice daily (Aqua Vitoe Laboratories, India). After debridement, lab results were HbA1c 37 mmol/mol, Hb 8.19 mmol/1, WBC 6.9 × 109/1, platelet count 134 × 109/1, serum creatinine 88.4 µmol/1 and CRP 20 mg/1.
Once the wound showed no signs of infection, autologous PRFM therapy was initiated. By the third week, there was a significant improvement in wound healing, and complete healing was achieved by the fifth week [Figure 5].
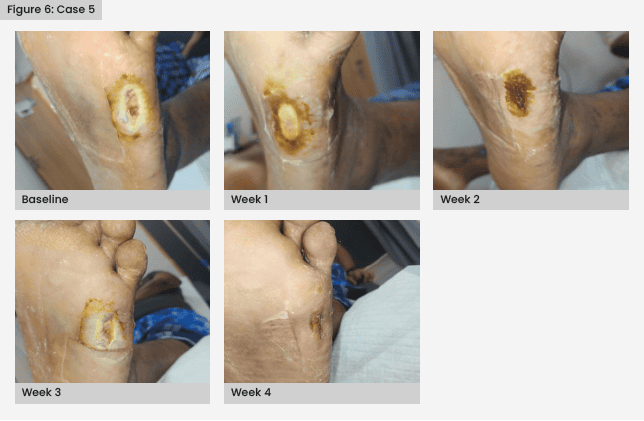
Case 5
A 30-year-old man with T2D diagnosed 7 months earlier presented with a left foot abscess (Wagner grade 2) and a foreign body.
The patient underwent left foot wound debridement and the foreign body, a small piece of glass, was removed. IV antibiotics were administered – linezolid 600 mg twice daily (Pure and Cure Healthcare, India), clindamycin 600 mg three times daily (Zen Pharma, India) and cefoperazone with sulbactam 3 g twice daily (Aqua Vitoe Laboratories, India).
At the time of debridement, lab results were HbA1c 117 mmol/mol, Hb 9.9 mmol/, platelet count 250 × 109/1, WBC 8.4 × 109/1, serum creatinine 97.24 µmol/l and CRP 90mg/l.
Once the wound showed no signs of infection, autologous PRFM therapy was initiated. By the third week, a significant improvement in wound healing was observed, and complete healing was achieved by the fifth week [Figure 6].
Discussion
The pathophysiology of DFUs is influenced by three main factors: neuropathy, arterial occlusion and trauma leading to secondary infection (Bandyk, 2018). DFUs present a significant healthcare challenge due to their extended healing periods, which frequently result in severe complications (Caruso et al, 2020). A key factor contributing to this delayed healing is chronic inflammation, characterised by dysregulated levels of matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of MMPs (Kartika et al, 2021). Therefore, it is crucial to expedite the healing process to effectively treat DFUs and reduce the risk of amputation.
PRFM is a concentrated form of platelets and growth factors derived from the patient’s own blood. The process involves centrifuging blood to separate plasma with a high concentration of platelets, fibrin and other bioactive molecules. This resulting matrix serves as a natural reservoir of growth factors, including platelet-derived growth factor, transforming growth factor-beta and vascular endothelial growth factor, which play pivotal roles in tissue repair and angiogenesis.
Using PRFM in DFU treatment stimulates cell proliferation and migration, promoting tissue regeneration. Transforming growth factor-beta aids in collagen synthesis, fostering wound closure and scar tissue formation. Vascular endothelial growth factor enhances angiogenesis, improving blood supply to the wound bed. Together, these factors create a synergistic environment conducive to accelerated healing.
Several studies have reported positive outcomes with PRFM. Recent research has shown that PRFM combined with hyaluronic acid significantly enhances angiogenesis and reduces inflammation in DFUs, leading to improved wound healing outcomes (Kartika et al, 2021). Another study highlighted the significant potential of PRFM in promoting the healing of chronic leg and foot ulcers, demonstrating a high percentage of complete ulcer healing with reduced pain and exudate (Rasajnam et al, 2022).
A study by Margolis et al (2001) involving 26,599 patients with DFUs, found that those treated with platelet-derived products tended to heal more quickly than those who did not receive these treatments. Despite the fact that the ulcers in the platelet-treated group were larger and deeper than those in the comparison group, these patients showed greater improvement by the end of 12 weeks. Similarly, Mazzucco et al (2004) found that treatment with platelet-rich gel significantly accelerated wound healing and reduced hospital stays.
Consistent with findings from other PRFM studies, we also observed successful wound healing in our case series.
Conclusion
The use of autologous PRFM presents a promising avenue for advancing the treatment of DFUs. The regenerative potential of PRFM, combined with its safety profile and patient-specific nature, positions it as a valuable addition to the armamentarium of healthcare professionals addressing the complexities of diabetic wound healing.
As research unfolds, the integration of PRFM into standard wound care protocols may signify a paradigm shift, offering renewed hope for improved outcomes and an enhanced quality of life for people with diabetes.
References:
