Barriers and enablers of strategies to reduce antibiotic prescribing in wound care: a narrative review
Received:
Accepted:
Published:
Authors: Kate Williams
Citation:
Williams K (2025) Barriers and enablers of strategies to reduce antibiotic prescribing in wound care: a narrative review. Global Wound Care Journal 1(2): 41–51
Conflicts of interest:
KW is on the Global Wound Care Journal editorial board; this did not influence acceptance.
Corresponding author:
Kate Williams, Accelerate CIC, St Joseph’s Hospice, Centenary Wing, St Joseph’s Hospice, Mare St, Hackney, London, E8 4SA, UK. Email: kate.williams121@nhs.net
DOI: https://doi.org/10.63896/gwcj.1.2.41
Background: Antimicrobial stewardship (AMS) is essential in the fight against antimicrobial resistance. National and international guidance advocate AMS with reference to specific infections but omit wound infection. Whereas international consensus documents recommend AMS practices in wound care, there are no clear metrics as to how well these recommendations have been implemented into clinical practice.
Objective: A critical appraisal of current evidence exploring the barriers and enablers of strategies to reduce inappropriate antibiotic prescribing in wound care.
Methods: A structured literature review was carried out using a narrative approach, with the structure of the PRISMA process. The study population consisted of patients in any healthcare setting over the age of 18 years. Searches were conducted in March 2024 using CINAHL, Embase, Emcare and MEDLINE. Searches dated back to 2015 when key national and international AMS guidelines were published. All studies were in English, measured the impact of interventions to reduce or optimise antibiotic prescribing, which included wounds, and incorporated rates of antibiotic prescribing as an outcome.
Results: Seven studies, dated from 2017 to 2021, met the inclusion criteria. Six were pre-/post-intervention studies with no control, and one was a case–control study. Five were based in acute hospital settings, and two were based in spinal cord injury rehabilitation settings. The thematic analysis highlighted six themes that enable strategies to reduce antibiotic prescribing in wound care: standardisation of care, leadership, education, culture of change, the role of the pharmacist and diagnostics.
Conclusion and implications for practice: Methodological weaknesses in the included studies suggest this review provides a platform for further study rather than having direct implications for practice. The inpatient setting for all studies limits the transferability to community care, where, in the UK, most people with wounds receive care and where most antibiotics are prescribed. Further mixed-methodological studies based in community care would add to the current body of evidence and address some of the complexities of AMS in wound care.
The term antimicrobial stewardship (AMS) has become firmly established in international public health guidance (European Union, 2017; World Health Organization [WHO], 2021). The intention is that all clinicians are “stewards” of antimicrobials, ensuring appropriate prescribing and judicious use of what is feared to be becoming a finite resource (WHO, 2021).
The WHO (2021) defines AMS as: “A coherent set of integrated actions which promote the responsible and appropriate use of antimicrobials to help improve patient outcomes across the continuum of care.” (WHO, 2021). Despite its eminence in global public health strategy, AMS has differing local interpretations and is still not something all healthcare professionals are aware of or see as part of their role (Dyar et al, 2017; Blaser et al, 2021; Ousey et al, 2022). The purpose of AMS is to combat the significant growing global concern surrounding antimicrobial resistance (AMR), with the number of antimicrobial-related deaths predicted to reach 10 million by 2050 (O’Neill, 2016).
More than 80% of antibiotics in the UK are prescribed in community settings (Lipsky et al, 2016; Public Health England, 2018), 50% of which are thought to be inappropriate prescriptions (Lipsky et al, 2016). Dolk et al (2018) reported that respiratory tract infections and urinary tract infections are common indications for antibiotics in community care, accounting for 46% and 22.7% of prescriptions, respectively, with skin and wound infections being the third most common, accounting for 16.3% of prescriptions in community care. Considering AMR and AMS in the context of wound care, an estimated 50% of wound patients receive antibiotics each year (Guest et al, 2020).
The International Wound Infection Institute (IWII) defines wound infection as: “The invasion of a wound by proliferating microorganisms to a level that invokes a local, spreading and/or systemic response in the host” (IWII, 2022). The probability of infection developing is influenced by the wound, patient and environmental factors.
Early diagnosis and treatment of local wound infection can prevent systemic infection and reduce the need for antibiotic prescribing (Öien and Forssell, 2013; IWII, 2022). AMS in wound care is complex (Doyle et al, 2022), with widespread use of topical (non-antibiotic) antimicrobials in wound care, such as silver and iodine (Cooper and Kirketerp-Møller, 2018; Edwards-Jones, 2020; Probst et al, 2022). Some authors have expressed concern about resistance to such antiseptics (Cooper and Kirketerp-Møller, 2018; Lipsky et al, 2016), although no resistance to povidone-iodine has been reported (Barreto et al, 2020), and there is little evidence of silver resistance in wound care (Percival et al, 2019). A systematic review concurred, reporting that there is little evidence that topical antimicrobials contribute to AMR, but highlighted that the role of topical antimicrobials in AMR continues to be an important area for investigation (Blackburn et al, 2022). There is undoubtedly evidence that the overuse of antibiotics contributes to AMR, with more prudent use of antibiotics needed to reduce drug resistance (O’Neill, 2016; Caputo et al, 2022). A global and national UK strategy has been developed to combat AMR (WHO, 2015; Department of Health and Social Care, 2022). However, given the absence of any UK government strategy on reducing antibiotic prescribing within wound care, a review of prior research is required to form the basis for future study of this complex field of practice.
Methodology
A narrative review was conducted, because undertaking a full systematic review was beyond the scope of this paper due to it being completed by a single researcher (Aveyard, 2014). Typically, narrative reviews are less structured and more descriptive than a systematic review (Aveyard, 2014; Grant and Booth, 2009); therefore, a structure was provided by using the PRISMA process with rigor in searches, synthesis and analysis (Grant and Booth, 2009).
Search strategy
The electronic databases searched were CINAHL, Embase, Emcare and MEDLINE. Embase was included to capture drug and pharmaceutical publications, given the focus on antibiotic prescribing within AMS. Grey literature was searched using Google and the social policy and practice database.
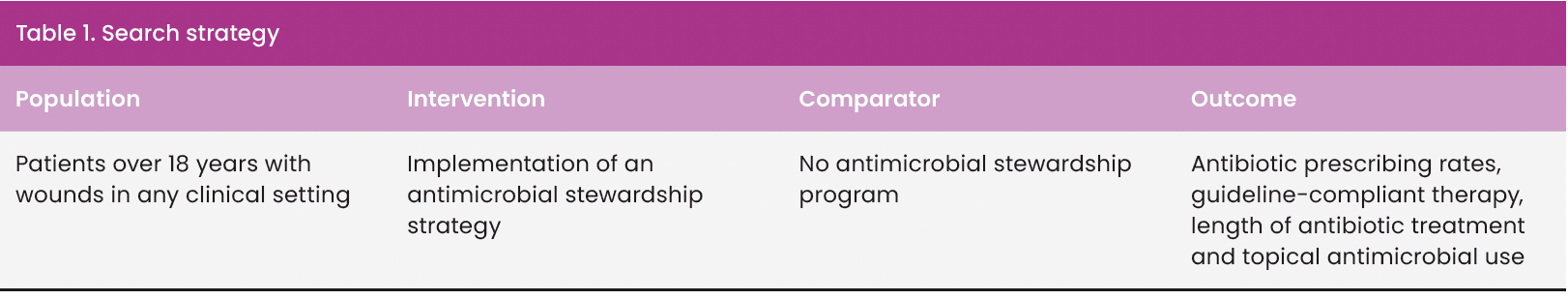
Searches dated back to 2015 to correspond with the WHO (2015) Global action plan on antimicrobial resistance and the NICE (2015) guideline, Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use. The search strategy was structured around the PICO framework [Table 1] to prompt key terms and maintain a focus (Bettany-Saltikov and McSherry, 2016; Gallagher Ford & Melnyk, 2019).
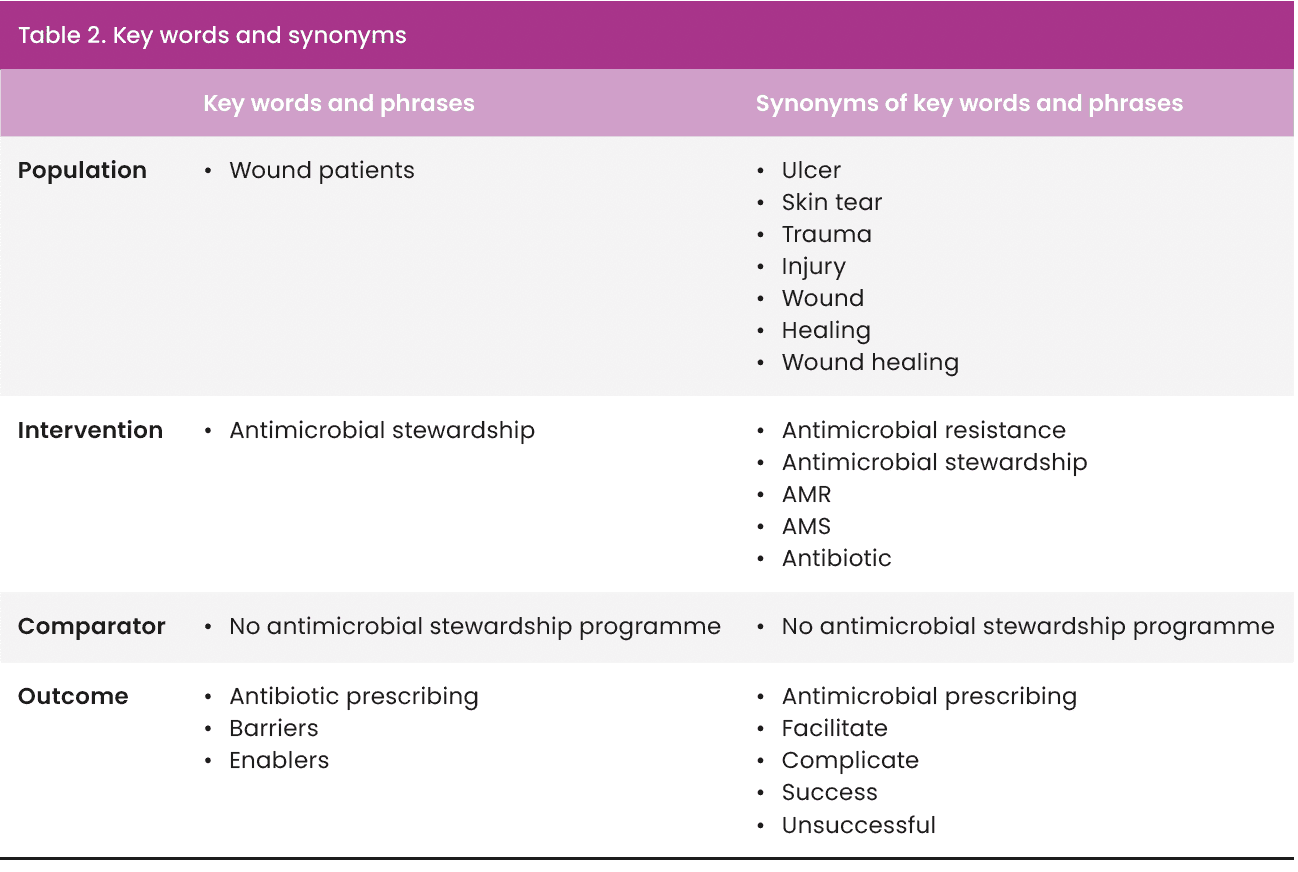
Key words and synonyms [Table 2] formed the basis of the search. Truncation and Boolean operators (‘AND’ and ‘OR’) were used to further refine the search (Dahlberg and McCaig, 2010). Proximity searches were also included to cover variations of phrases (Booth et al, 2022).
Study selection process
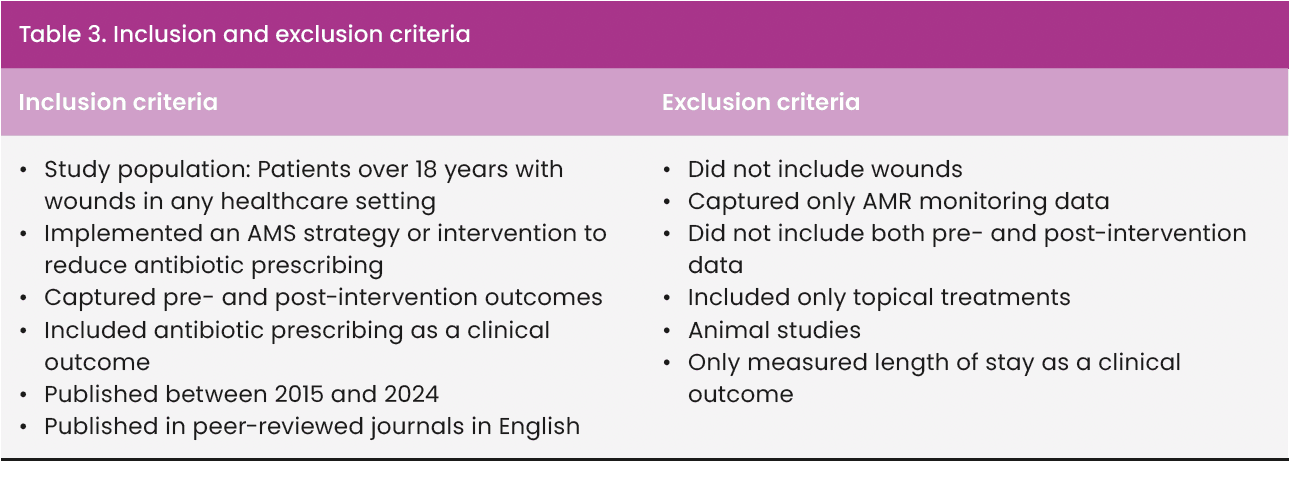
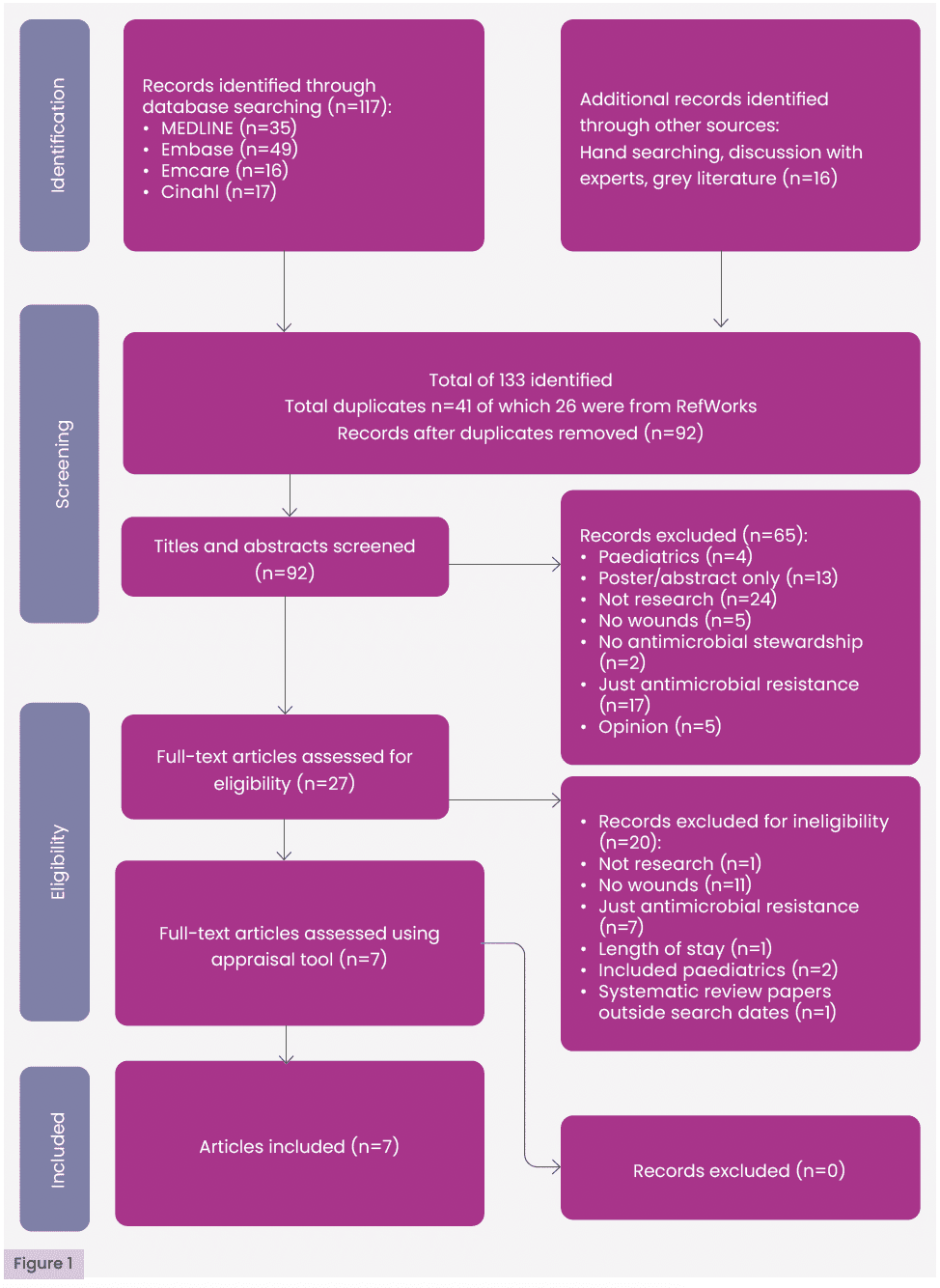
Duplicates were removed using RefWorks and manual screening. Titles and abstracts were then screened based on the inclusion and exclusion criteria [Table 3]. Full texts of potentially relevant studies were assessed for eligibility. Only studies meeting all criteria were included in the final review. Quality appraisal was subsequently conducted. A summary of the selection process is presented in the PRISMA flow diagram [Figure 1].
Quality appraisal
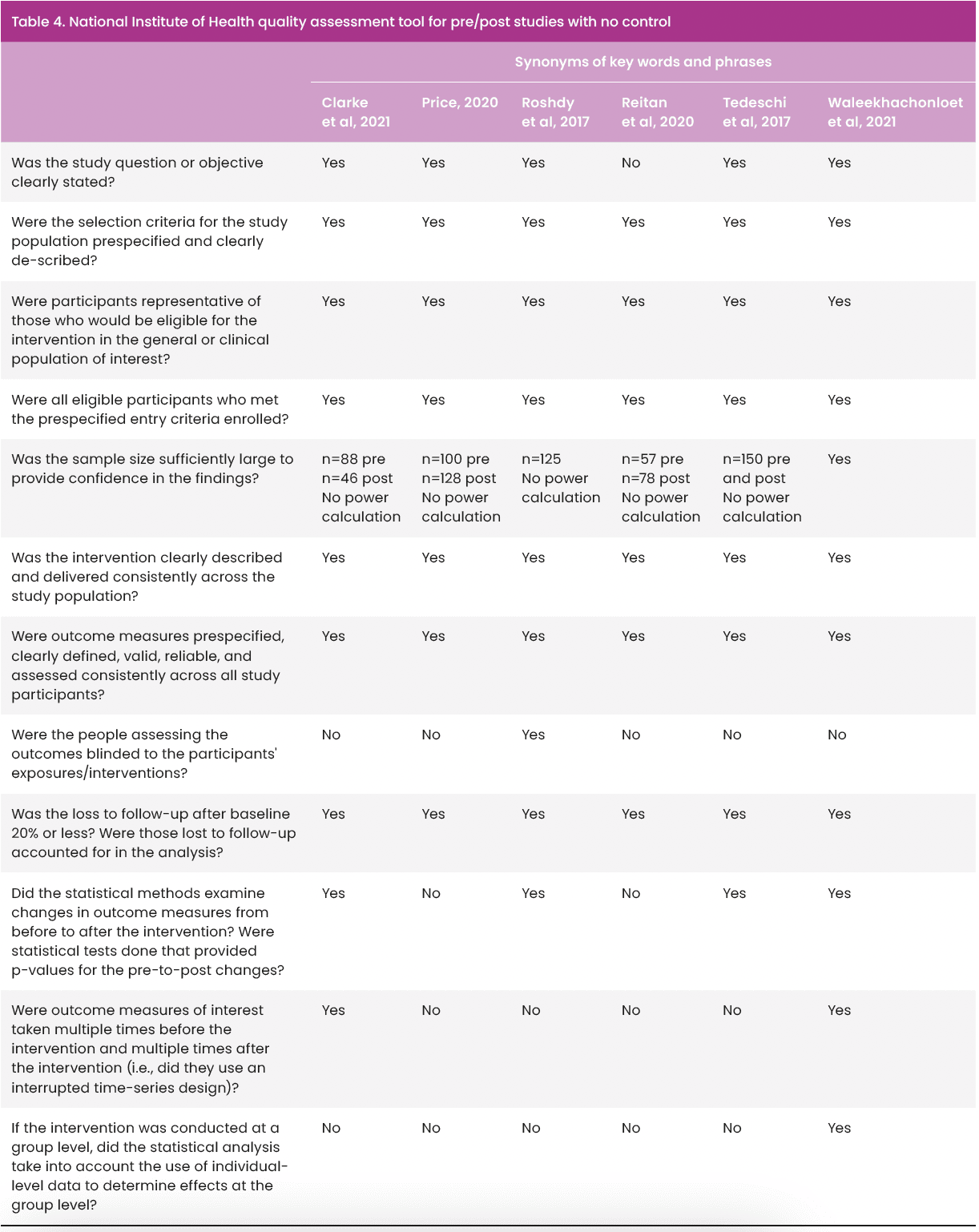
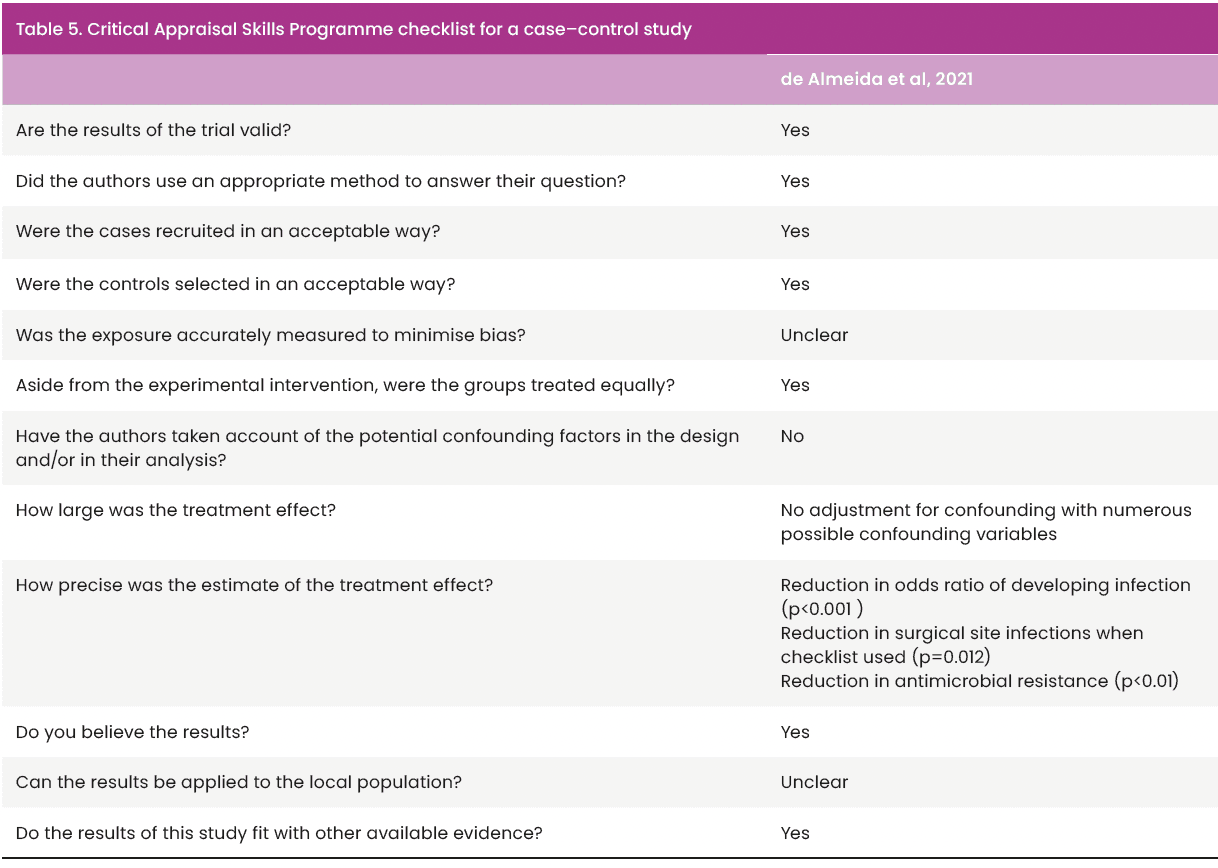
Checklists were used to reduce the subjective nature of appraisal by one researcher (Aveyard, 2014; Booth et al, 2022). Papers that were quasi-experimental before and after studies with no control were appraised using the National Institutes of Health (NIH) quality assessment tool for pre/post studies with no control [Table 4]. The remaining study was a cohort study; therefore, the Critical Appraisal Skills Programme (Critical Appraisal Skills Programme Checklist, 2022) tool for cohort studies [Table 5] was used to structure quality appraisal.
Data extraction
All data relevant to barriers and enablers of strategies to reduce antibiotic prescribing in wound care were extracted to reduce outcome selection bias (Booth et al, 2022). Homogeneity and heterogeneity of studies were noted to shape data synthesis and analysis (Bettany-Saltikov and McSherry, 2016; Booth et al, 2022).
Data synthesis
Included studies were of similar design, although homogeneity of aims and outcomes was lacking. Heterogeneity was explored using descriptive statistics; however, given the variation in outcomes across the studies, thematic analysis (Braun and Clark, 2006) was most appropriate. Narrative around each theme was complemented with tabular synthesis and charting of relevant data (Booth et al, 2022). Some subgroup analysis was considered where studies shared similar characteristics, but this was limited due to the heterogeneity of the studies.
Results
Searches identified 133 papers. Of these, 41 were excluded as they were duplicates, 85 papers were excluded as they did not meet the inclusion criteria, and the remaining seven papers were assessed using NIH or Critical Appraisal Skills Programme critical appraisal tools and deemed to be of sufficient methodological standard to be included in the review. No further papers were excluded. A summary of the process can be seen in the PRISMA flow diagram [Figure 1].
Study characteristics
The seven papers included in this review (Roshdy et al, 2017; Tedeschi et al, 2017; Price, 2020; Reitan et al, 2020; Clarke et al, 2021; de Almeida et al, 2021; Waleekhachonloet et al, 2021) are summarised in Appendix 1. All were published in peer-reviewed journals. Geographical locations varied, with two studies from low-middle-income countries (de Almeida et al, 2021; Waleekhachonloet et al, 2021). This holds significance, due to a 77% increase in antibiotic use across low–middle-income countries from 2000 to 2015 (Blaser et al, 2021). With regards to healthcare settings, four were based in acute care (Roshdy et al, 2017; Reitan et al, 2020; de Almeida et al, 2021; Waleekhachonloet et al, 2021), two in spinal cord injury rehabilitation facilities (Tedeschi et al, 2017; Clarke et al, 2021) and one in a hospital clinic (Price, 2020). No studies were based in community care settings. This may limit the transferability of the findings of this review to countries, such as the UK, where wounds are largely managed in the community (Guest et al, 2020). Five studies reported study population demographics (Roshdy et al, 2017; Price, 2020; Reitan et al, 2020; Clarke et al, 2021; de Almeida et al, 2021), which were noted to be similar in terms of age range and gender. Comorbidities were poorly documented, with only one paper (de Almeida et al, 2021) capturing these data and performing subgroup analysis according to comorbidities. Price (2020) focused on patients with a diabetic foot ulcer, but did not comment on diabetes control or other comorbidities. Reitan et al (2020) presented data specific to people with diabetes, but did not specify whether diabetes was well controlled within the study population. Given the significant impact of comorbidities on wound healing, the risk of wound infection (Doyle et al, 2022; Malone and Schultz, 2022), the omission of comorbidities inevitably affects the validity of each study.
Six studies (Tedeschi et al, 2017; Roshdy et al, 2017; Price, 2020; Reitan et al, 2020; Clarke et al, 2021; Waleekhachonloet et al, 2021) were quasi-experimental pre/post-studies with no control group. Such studies are often used to measure the impact of health interventions but carry a significant risk of bias when measuring efficacy, so careful interpretation of results is essential (Centre for Reviews and Dissemination, 2009; Goodacre, 2015; Schaffer et al, 2021). Bias is of particular concern in five studies (Roshdy et al, 2017; Tedeschi et al, 2017; Price, 2020; Reitan et al, 2020; Clarke et al, 2021) because pre- and post-intervention data capture does not account for any pre-existing trends; however, the interrupted time series analysis used by Waleekhachonloet et al (2021) considers baseline trends over time thereby providing a robust design for measuring health interventions in the absence of control groups (Goodacre, 2015; Schaffer et al, 2021).
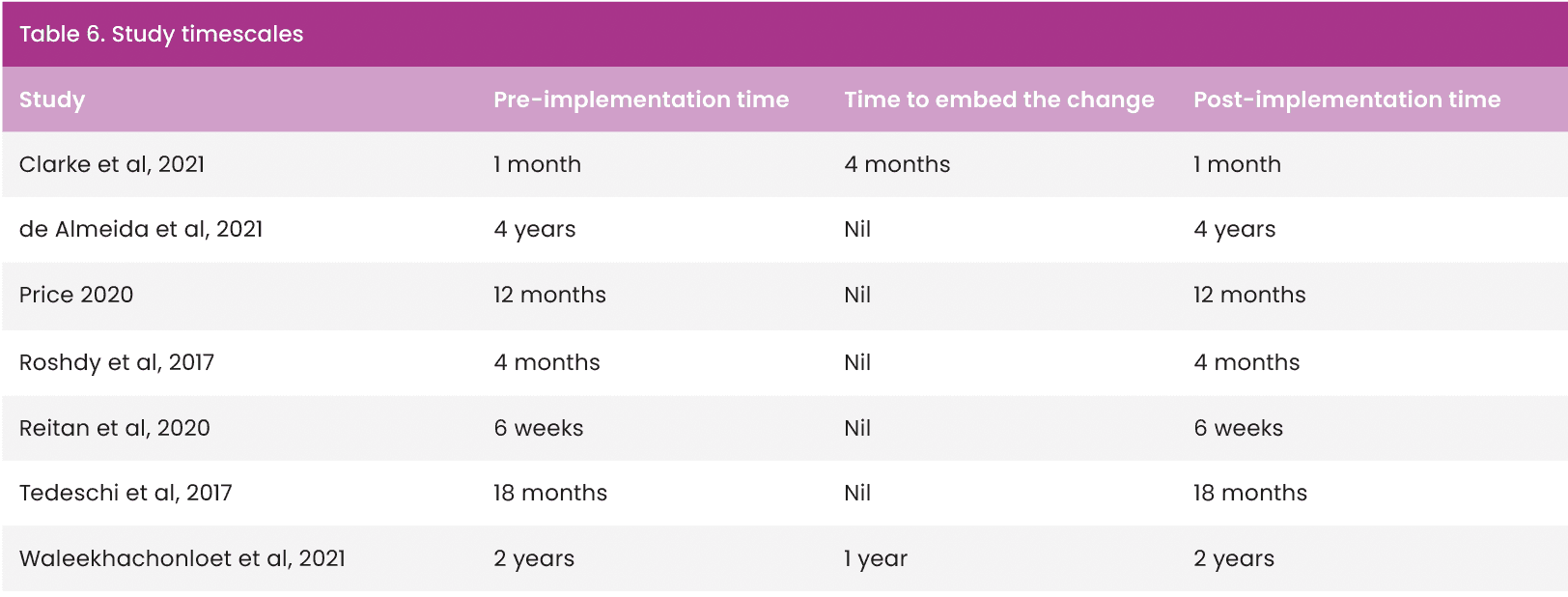
Study timescales ranged from 6 months to 4 years [Table 6]. Five studies (Roshdy et al, 2017; Tedeschi et al, 2017; Price, 2020; Reitan et al, 2020; de Almeida et al, 2021) did not report time to embed the change in practice; therefore, not recognising that any process of change or implementation requires a period of settling to become normal practice and demonstrate real change (Eaton, 2014). This is a recognised disadvantage to pre-/post-studies, which do not allow time to embed change, nor continue for sufficient time to observe if effects of interventions are sustained (Goodacre, 2015). Clarke et al (2021) acknowledged this and posed a similar argument to justify a 4-month period to embed their intervention, yet had the shortest pre- and post-implementation time of all the studies. This further affects the transferability and generalisability of all the study outcomes, the exception being Waleekhachonloet et al (2021), whose total study timescale was 5 years.
Sample sizes varied and were reported as: number of prescribing events (Clarke et al, 2021); wounds (Price, 2020); patients (Roshdy et al, 2017; Reitan et al, 2020; de Almeida et al, 2021); beds (Tedeschi et al, 2017); and hospitals (Waleekhachonloet et al, 2021) so direct comparison was not possible. No authors included a power calculation to determine sample size, which could have resulted in results not being statistically significant where they should be (Greenhalgh, 2019).
Findings
During the data extraction process, six themes emerged as potential barriers and/or enablers of strategies to reduce antibiotic prescribing in wound care:
- Standardisation of care
- Leadership
- Education
- Culture of change
- Role of the pharmacist
- Diagnostics.
Standardisation of care
Standardisation refers to guidelines and pathways. Concordance with prescribing guidance was a common theme, with guideline-concordant therapy as an outcome measure in two studies (Roshdy et al, 2017; Clarke et al, 2021). Other approaches to standardisation included implementing computer-based guidance with a supporting clinical pathway (Roshdy et al, 2017). The significant decrease in antibiotic prescribing trends demonstrated by Waleekhachonloet et al (2021), based in Thailand, which, until the point of the study, had no national AMS guidance in place, suggests standardisation through prescribing guidelines is certainly an enabler to AMS strategies in wound care.
Leadership
In this context, leadership refers to an individual or a multidisciplinary team (MDT) of clinicians who lead an AMS initiative. Clarke et al (2021) achieved an increase in guideline-compliant prescribing (p<0.001) and a 9% reduction in antibiotic prescribing for wounds (no p-value given) by implementing consultant- and pharmacist-led ward rounds. Tedeschi et al (2017) implemented an infectious diseases consultant ward round, which led to a decrease in antibiotic consumption (p<0.001). Leadership was a narrative theme in other papers (Roshdy et al, 2017), but was linked with leading education on prescribing guidelines rather than a clinical leadership role. Despite this, and despite a lack of subgroup analysis specific to wounds in the above studies, leadership and MDT working emerged as an enabler of strategies to reduce antibiotic prescribing.
Education
Education was integral to four studies (Roshdy et al, 2017; Tedeschi et al, 2017; Reitan et al, 2020; Waleekhachonloet et al, 2021), in that each study implemented a change that required education for clinicians. Any correlation between education and clinical outcomes was not measured in any study, and as such is assumed. Roshdy et al (2017) emphasised the barrier of lack of education in relation to achieving any of the study outcomes, but again, this was not measured. All four studies suggest (but cannot demonstrate) that education is an enabler of strategies to reduce antibiotic prescribing, arguing that education facilitates effective implementation of prescribing guidelines and that lack of access to education creates a barrier to change.
Culture of change
Positive change culture was acknowledged as an enabler of strategies to reduce antibiotic prescribing in four studies (Tedeschi et al, 2017; Clarke et al, 2021; de Almeida et al, 2021; Waleekhachonloet et al, 2021), although this was an unexpected finding. Prescribing behaviours were highlighted as potential barriers if change processes were not managed effectively (Tedeschi et al, 2017; Clarke et al, 2021; Waleekhachonloet et al, 2021). Each study identified attitudes towards change as either a barrier or an enabler, describing senior MDT role modelling and leadership as key to the success of any change in practice or clinical outcome. The culture of change was not measured or directly linked to clinical outcomes in any study.
Role of pharmacist
Three studies (Roshdy et al, 2017; Clarke et al, 2021; Waleekhachonloet et al, 2021) reported involvement of a pharmacist as an enabler to an effective antibiotic reduction strategy. Each study emphasised the positive role of the pharmacists in reviewing prescriptions and discussing antibiotic choices, doses, and duration of therapy. The role of pharmacists was not an intentional outcome measure in any studies; however, the impact of a pharmacist on any of the defined outcomes is not quantified. The role of the pharmacist is a more common theme in the narrative surrounding the results, which emerged as an enabler of strategies to reduce antibiotic prescribing in wound care.
Diagnostics
The narrative from Waleekhachonloet et al (2021) reported that antibiotics may be prescribed for contaminated and clean wounds. This is a significant barrier to antibiotic reduction strategies, as contaminated wounds do not warrant treatment with antibiotics or antiseptics, as contamination does not elicit a host response or cause a delay in healing (IWII, 2022). Reitan et al (2020) found that implementation of a tool to aid diagnosis of wound infection led to a statistically significant (p=0.049) reduction in antibiotic prescribing for “infected ulcer”. However, overall antibiotic prescribing did not reduce significantly (p=0.163). Price (2020) reported that introducing fluorescence imaging in a diabetic foot clinic led to a 33% reduction in antibiotic prescribing. No statistical analysis was reported. The results are possibly clinically significant, but whether this is due to more accurate diagnosis using fluorescence or an incidental improvement from a renewed focus on accurate diagnosis within the clinic is unknown.
Discussion
The themes presented are consistent with recommendations in international guidance specific to wound care (Probst et al, 2022). Numerous authors reference the need for standardisation of care, MDT working, and diagnostics (Lipskey, 2016; Cooper and Kirketerp-Møller, 2018; Uçkay et al, 2019; Edwards-Jones, 2020; Ousey and Sussman, 2021; Caputo et al, 2022; IWII, 2022).
In terms of leadership, papers within this review refer to pharmacists and infectious diseases or spinal consultants (Tedeschi et al, 2017; Clarke et al, 2021), being clinicians best placed to drive acceptance and integration of strategies to reduce antibiotic prescribing. The importance of pharmacists as members of the wound care MDT has been discussed widely, with some authors suggesting that pharmacists are ideally placed to lead strategies to reduce antibiotic prescribing in wound care (Ousey and Sussman, 2021). Interestingly, the role of nurses as leaders of AMS strategies was not identified in any studies identified in this review. This is despite nurses being recognised as key members of the MDT in wound care (Rippon et al, 2021).
Any AMS strategy in wound care is complex, and the time commitment required to work as an MDT needs to be acknowledged (Doyle et al, 2022). Uçkay et al (2019) identified barriers to MDT working in this field, although these were identified prior to the COVID-19 pandemic. The acceptance of online meetings and video consultations could enable MDT working and leadership in ways that research pre-2020 could not have foreseen. Although not specific to antibiotic prescribing, Mohamedbhai et al (2021) found most MDT members would welcome a hybrid approach to meetings where the enhanced teamwork and training opportunities in face-to-face meetings can be combined with the flexibility of virtual attendance.
Education emerged as an enabler of strategies to reduce antibiotic prescribing in wound care, but the delivery of education varied enormously. Global and national documents all refer to a need for education (Pollack and Srinivasan, 2014; NICE, 2015; Public Health England, 2015; WHO, 2021; Probst et al, 2022), but reflect the findings of this review in that each has a different education focus, be it awareness, education and training (Waleekhachonloet et al, 2021; WHO, 2021), or public engagement (Public Health England, 2015). Doyle et al (2022) support education of professionals, but also highlight the importance of including patient education to include how to identify an infected wound and when to seek advice from a healthcare professional. Engaging health professionals and the public may be challenging if there is little incentive or appetite for change. Previous systematic reviews have supported the concept of change culture as a barrier to wider AMS strategies (Charani et al, 2011) and emphasise the paucity of high-quality evidence on which antibiotic-related behaviour change is based. The quality premium financial incentive implemented in England in 2015 (NHS England, 2015) reported to have achieved a reduction in antibiotic prescribing for respiratory infections (Bou-Antoun et al, 2018), but the effect on antibiotic prescribing for wounds remains unknown.
None of the studies in this review considered prescribers’ fear of not prescribing (Cooper and Kirketerp-Møller, 2018). Analysis of the complexities of behaviour change among prescribers is beyond the scope of this review; however, the management of behaviour change has emerged as both a barrier and an enabler of strategies to reduce antibiotic prescribing in wound care.
Diagnostics
Diagnosis of wound infection is primarily through clinical findings, with support from microbiological data (Lipskey et al, 2016; Malone and Schultz, 2022). Only two papers (Price, 2020; Reitan et al, 2020) had a focus on accurate diagnosis of infection, although both had significant methodological flaws. These included a short study period (Reitan et al, 2020), retrospective analysis, lack of power analysis in the sampling (Price, 2020; Reitan et al, 2020) and the absence of statistical analysis (Price, 2020). Diagnostics is a focus in numerous publications specific to AMS in wound care (Lipskey et al, 2016; Uçkay et al, 2019; Ahmed et al, 2021; Ousey and Sussman, 2021; Rippon et al, 2021; Malone and Schultz, 2022; Probst et al, 2022). Diagnosing wound infection can be difficult, and microbiological sampling does not help (Edwards-Jones, 2018; Malone and Schultz, 2022). There is a reliance on the expertise of the clinician to diagnose infection (Blackburn et al, 2024). The absence of a standard test to identify wound infection complicates this further (Roberts et al, 2017), with some studies (Price, 2020; Reitan et al, 2020) lacking methodological quality to support widespread change. Despite an abundance of publications emphasising the importance of accurate, prompt diagnosis of wound infection, uncertainty remains (Cooper and Kirketerp-Møller, 2018). This apparent gap between research and clinical practice requires further investigation for clinicians to understand the importance of accurate, prompt diagnosis of wound infection.
Study limitations
Several important limitations need to be considered. Only English language papers were retrieved despite AMR being of global concern, and only four papers (Roshdy et al, 2017; Price, 2020; Reitan et al, 2020; de Almeida et al, 2021;) explored wound infections. Throughout this review, direct comparison was prevented by the heterogeneity of outcomes across papers, and methodological flaws limit the validity of the review findings. Flaws included bias from the pre/post with no control design; study periods that ranged from 8 weeks to 4 years, and a lack of power analysis of sample sizes.
Conclusion
The methodological weaknesses in the included studies suggest this paper provides a platform for further study rather than having direct implications for practice. Methodological improvements must be made with future studies specifying the rationale, setting, aims and features of the intervention clearly (Schweitzer et al, 2020). The acute medical focus of papers in this review further limits any influence on practice in the UK, where wound care is mostly led by nurses, in community settings, with an increasing reliance on the non-registered clinical workforce (Ousey and Atkin, 2022). To further understand barriers and enablers of strategies to reduce inappropriate antibiotic prescribing, there needs to be a better understanding of how these apply in nurse-led services, with a large, unregistered workforce.
