Use of DACC-coated wound dressings in the reduction of surgical site infection: a systematic review and meta-analysis
Received:
Accepted:
Published:
Authors: Mark G Rippon, Alan A Rogers, Karen Ousey and John Stephenson
Citation:
Rippon MG, Rogers AA, Ousey K, Stephenson J (2025) Use of DACC-coated wound dressings in the reduction of surgical site infection: a systematic review and meta-analysis. Global Wound Care Journal 1(1): 24-30
Conflict of interest
Karen Ousey is an employee of the journal publisher, Omniamed Communications; this did not affect peer review. All other authors have no conflicts of interest to declare.
Ethical statement
No ethical approval was required as this is a systematic review with meta-analysis.
Corresponding author
Mark G Rippon, University of Huddersfield, Queensgate, Huddersfield, UK, HD1 3DH. Email: markgeoffreyrippon@gmail.com
DOI: https://doi.org/10.63896/gwcj.1.1.24
Aim: To assess the evidence from randomised controlled trials and quasi-experimental studies that have studied the use of DACC-coated dressings in reducing surgical site infection (SSI). Methods: A systematic review with meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement standards. Randomised controlled trials (RCT) and quasi-experimental studies comparing DACC-coated dressings against a non-DACC-coated dressing were considered for inclusion. All studies meeting eligibility criteria were assessed for risk of bias. A random effects meta-analysis was conducted on the SSI outcome, with a sensitivity analysis conducted to assess influence of individual studies. Study homogeneity and the relationship between control and intervention treatments was explored via Galbraith and L’Abbé plots. Results: Five studies were identified for inclusion. All were considered low or medium risk of bias. The synthesised odds ratio for SSI was 0.59 (95% CI [0.46–0.75]); hence, odds of SSI were almost halved in patients treated with DACC-coated dressings. The quality of the evidence as rated as high, with results indicating high levels of consistency, precision and directness, in conjunction with the risk of bias findings. Conclusion: DACC-coated dressings are effective in reducing SSI after surgery.
Surgery, by its invasive character, can enable the transfer of pathogens that enter the body through incisions made during surgery (Bath et al, 2022), possibly resulting in postoperative surgical site infection (SSI). An SSI occurs near or at the incision site, and/or deeper underlying tissue spaces and organs within 30 days of a surgical procedure or within 90 days for implanted prosthetics (Borchardt and Tzizik, 2018; Pinchera et al, 2022).
SSIs are a major cause of morbidity and mortality, and the risk of mortality in patients with SSI is between 2 and 11 times higher than in patients with no SSI (Ban et al, 2016). They are one of the most reported infections globally (Mengistu et al, 2023). SSIs are also associated with increased complication rates, hospital stay/readmission, reduction in quality of life and higher treatment costs; thus, they are a significant financial burden on healthcare providers (Bath et al, 2022; Pinchera et al, 2022).
A cornerstone of prevention and treatment of SSIs is the use of antibiotics (Alsaeed et al, 2022). However, antibiotic resistance is one of the biggest threats to global health, food security and development, according to the World Health Organization, and it has become a leading cause of death globally (World Health Organization, 2023; Bishen, 2024).
To reduce the risk of antimicrobial resistance (AMR), it is imperative to establish alternatives to antibiotics. These include antiseptics, such as iodine, silver and polyhexamethylene biguanide, but these products also have problems associated with toxicity and the development of AMR (Rembe et al, 2016; Hosny et al, 2019; McNeilly et al, 2021; Romano et al, 2022; Vishwanath et al, 2022).
Dialkylcarbamoylchloride (DACC)-coated wound dressings use a hydrophobic wound contact layer that binds bacteria and removes them from the wound bed and are instrumental in reducing AMR in SSIs (Rippon et al, 2021). DACC-coated dressings have been used frequently for the successful prevention and treatment of SSIs (Stanirowski et al 2016a, 2016b; Rippon et al, 2023).
The aim of this systematic review and meta-analysis was to assess the evidence from randomised controlled trials and quasi-experimental studies that have studied the use of DACC-coated dressings in reducing SSI.
Methods
A systematic review with meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement standards.
Inclusion and exclusion criteria
Randomised controlled trials (RCT) and quasi-experimental studies were considered for inclusion if they compared the use of DACC-coated dressings as part of a treatment regimen for reduction of SSIs against a suitable comparator dressing (non-DACC-coated). No constraints were placed on language.
Search strategy
The search strategy was based on the planned Population, Intervention, Comparison and Outcomes (PICO) elements:
- P (population) — people who have undergone a surgical procedure .
- I (intervention) — DACC-coated dressing.s
- C (comparison) — standard care.
- O (outcomes) — SSI incidence within 30 days of procedure.
The literature was searched using search terms identified by the authors using the flowing key words: ‘dacc’, ‘dialkylcarbamoyl chloride’, ‘dialkyl-carbamoyl-chloride’, and ‘Sorbact’. The search terms were combined with ‘OR’ to create the search strategy: ‘dacc OR “dialkylcarbamoyl chloride” OR “dialkyl-carbamoyl-chloride” OR Sorbact’. PubMed and Scopus were searched, with hand searching of relevant references of included studies between January 2000 and January 2025. Two authors (AR and MR) independently assessed the titles and abstracts of all potentially relevant studies. In the case of any disagreement over eligibility, a third author (KO) reviewed the papers independently to facilitate a consensus decision.
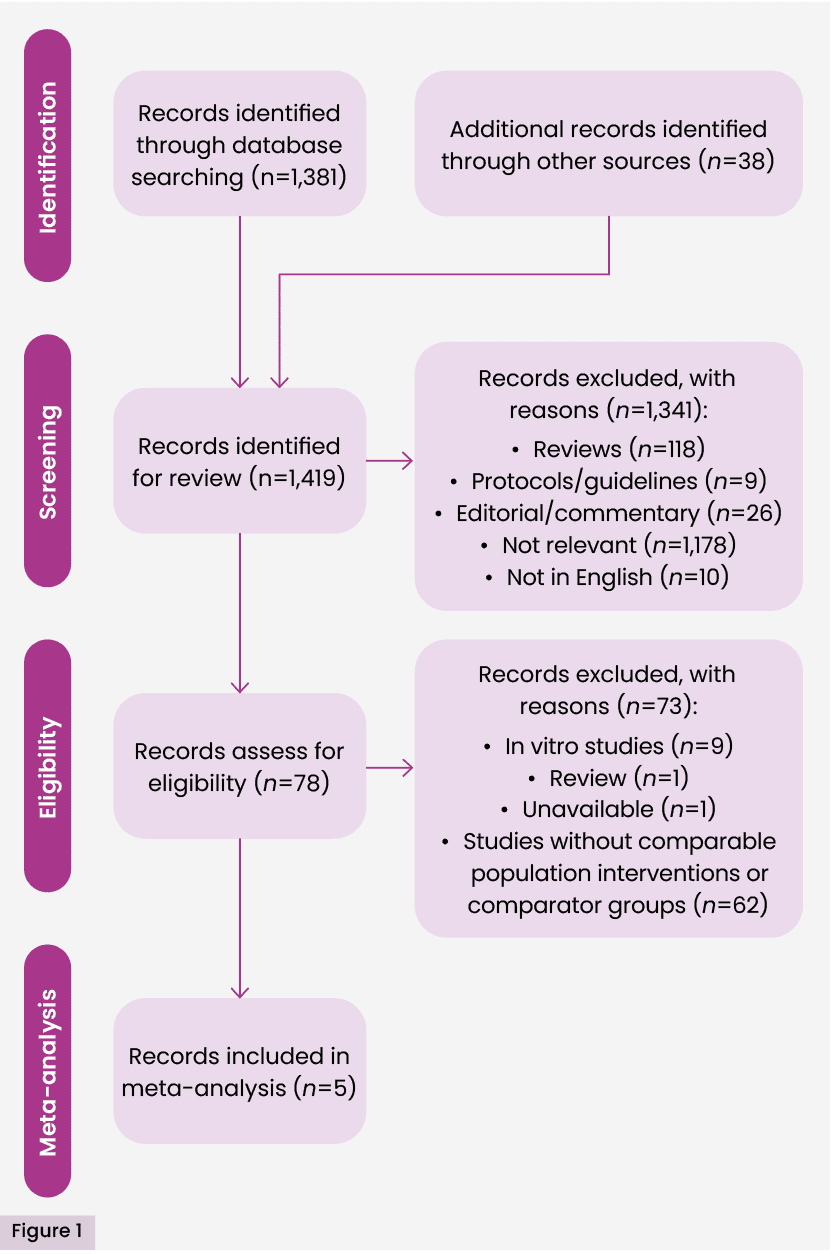
The initial search identified 1,419 studies; 78 studies were assessed as eligible for full text review. Two authors (MR and AR) independently conducted full-text reviews of all studies identified from the abstract screening. In the case of any disagreement over eligibility, a third author (KO) reviewed the papers independently to facilitate a consensus decision. Studies excluded did not explore acute surgical wounds. Five studies (Stanirowski et al, 2016a, 2016b; Bua et al, 2017; Totty et al, 2019; Magro, 2023) were included in the meta-analysis with the outcome of incidence of SSI within 30 days of procedure. Bua et al (2017) performed a quasi-experimental study (i.e. in which participants are allocated to groups by a non-randomised method). In this research, allocation was by order of entry into the study. Magro (2023) was badged as an audit, but is equivalent to a quasi-experimental study, insomuch as it included a comparative analysis of groups with non-random allocation. In this case, groups were defined as current patients and historical controls. The remaining included studies were randomised controlled trials. The detailed process of selection is presented in Figure 1.
Risk of bias assessments
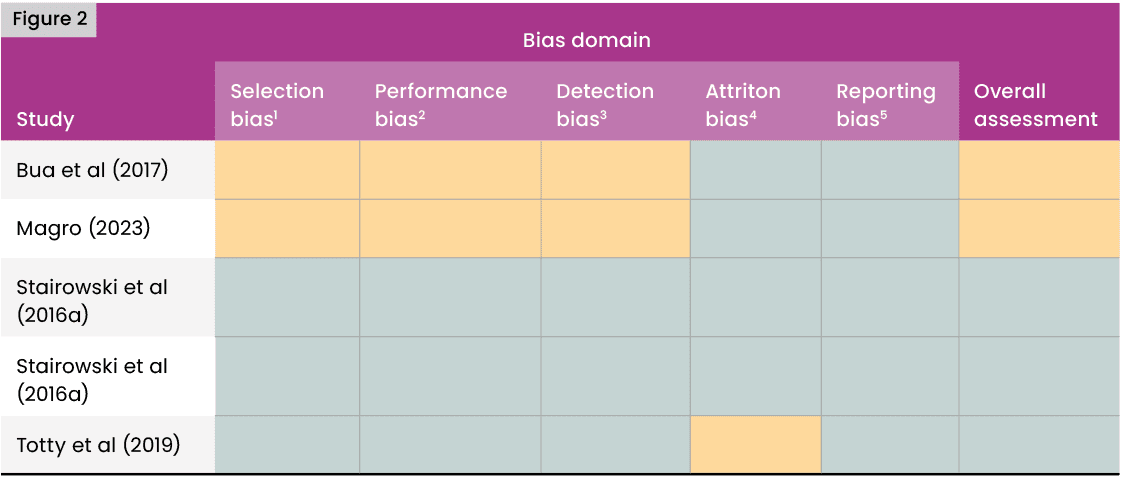
Risk of bias assessment for all studies meeting eligibility criteria was conducted using the Cochrane risk of bias tool (Higgins et al, 2011), classifying risk of bias in five domains: selection bias, performance bias, detection bias, attrition bias and reporting bias, with an overall assessment of bias made in each case by consideration of results in each domain. Results were summarised in a traffic light table. Risk of bias was assessed independently by two authors (MR, KO), with any disagreements resolved by consensus.
Meta-analysis methods
Meta-analyses were performed on randomised controlled trials (RCTs) and quasi-experimental studies of comparable outcomes featuring in two or more studies based on sufficiently comparable populations interventions and comparator groups.
Incidence of SSIs within 30 days of procedure was designated the primary outcome. All other outcomes featured in the identified RCTs were designated as secondary outcomes. Meta-analyses were not conducted on secondary outcomes due to no more than one study reporting each identified outcome.
A random effects meta-analyses was proposed for the SSI outcome using restricted maximum likelihood estimation methods. The random effects model, yielding conservative estimates compared to models based on fixed estimation effects, was chosen to reflect recognised clinical and methodological heterogeneity across included studies with respect to the SSI outcome. As a binary measure, the synthesised estimate was based on Mantel–Haenszel odds ratios (odds of SSI following treatment with DACC-coated dressings, compared to odds of SSI following treatment with standard non-DACC-coated dressings) and associated 95% confidence intervals (CIs). This statistic was based on numbers of reported events and non-events in both treatment groups in study; either reported directly or with event numbers calculated from reported percentages.
Meta-analysis reporting of the SSI outcome was via the construction of a forest plot, reporting synthesised estimates and associated 95% CIs, a Z-test for the estimated effect and heterogeneity statistics; including results from Cochran’s Q test for heterogeneity, the I2 statistic (proportion of variation across studies ascribed to heterogeneity) and the τ2 statistic (an estimate of between-study variance). Heterogeneity was further explored via a Galbraith plot, and a L’Abbé plot was used to visually explore the relationship between treatment effects and event rates across studies.
A sensitivity analysis was conducted on the meta-analysis to assess the robustness of the derived estimates via an influence plot. Small study effect-related bias (including publication bias) via a funnel plot was proposed of a primary outcome, subject to a minimum of 10 studies being included in the analysis; but not undertaken due to a paucity of included studies. No subgroup analyses were proposed or conducted. All significance testing was conducted at the 5% level of significance.
Assessment of evidence quality
The quality of the evidence was informally rated, based on the Cochrane GRADE framework; assessing the baseline study quality and the potential for downgrading due to risk of bias, inconsistency, imprecision, indirectness and publication bias. A decision threshold of 20% reduction in odds of SSI incidence was used to assess precision of evidence.
Results
Risk of bias
The quasi-experimental studies of Bua et al (2017) and Magro (2023) were judged to be at unclear risk of selection and performance bias because group allocations were not concealed and studies were not blinded. The study of Totty et al (2019) was subject to slight differential attrition and included errors in the patient flowchart, and was hence considered to be at medium risk of attrition bias. No selective reporting of outcomes was observed in any study. The overall assessments were low risk of bias (green) in the randomised controlled trials and unclear risk of bias (amber) in the quasi-experimental studies. Results are summarised in Figure 2.
Meta-analysis results
Five studies (Bua et al, 2017; Magro 2023; Stanirowski et al, 2016a, 2016b; Totty et al, 2019) were included in the meta-analysis of SSI, with 5,840 participants in total. Two studies (Bua et al, 2017; Totty et al, 2019) reported SSI incidence following vascular surgery. All other included studies reported SSI incidence following caesarean section. Three studies (Stanirowski et al, 2016a, 2016b; Totty et al, 2019) were randomised controlled trials; two studies (Bua et al, 2017; Magro 2023) were quasi-experimental studies or could be considered to be equivalent to quasi-experimental studies.
Two studies (Magro 2023; Stanirowski et al, 2016a) reported a significant reduction in the level of SSIs in patients undergoing C-section or vascular surgery using DACC-coated dressings. Three studies (Stanirowski et al, 2016b; Bua et al, 2017; Totty et al, 2019) reported a non-significant significant reduction in the level of SSIs with DACC. No studies reported an increase in the level of SSIs with DACC dressings.
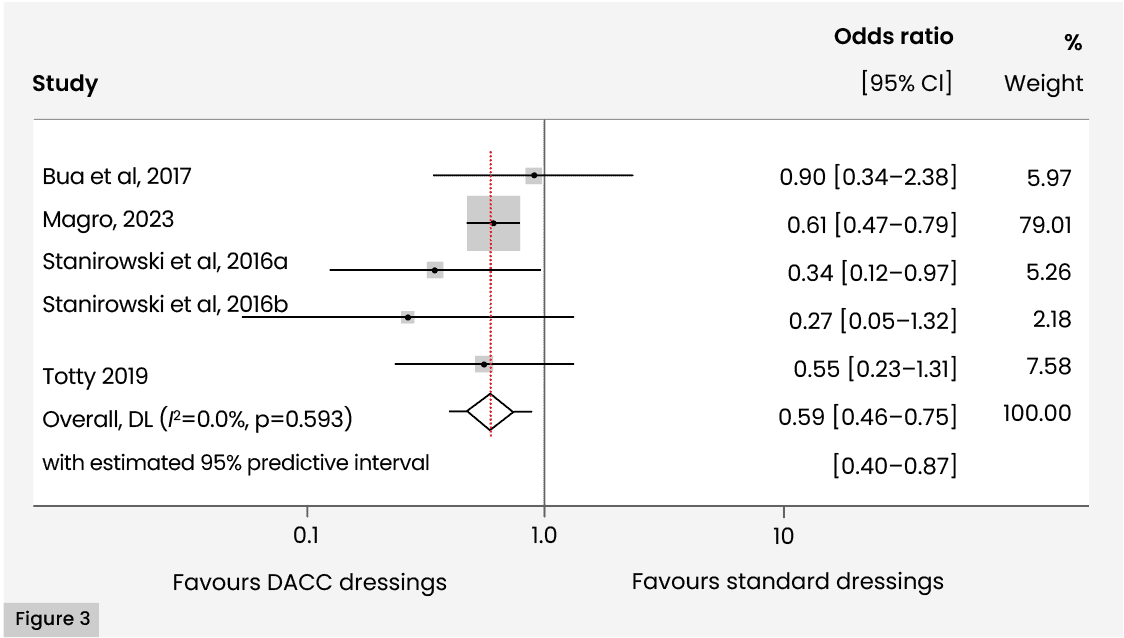
A meta-analysis of the primary outcome of SSI revealed that a synthesised estimate of the Mantel-Haenszel odds ratio for the odds of SSI in patients undergoing C-section or vascular surgery, comparing intervention to control treatment, 0.585 (95% CI [0.462–0.741]); i.e. odds of SSI in patients using DACC-coated dressings were 58.5% those of SSI in patients using standard (non-DACC-coated) dressings. A 95% predictive interval for the estimate was given by (0.40, 0.87). A Z-test of the effect revealed strong evidence for a non-zero effect (Z=4.45; p<0.001). Individual estimates for the odds ratio ranged from 0.265 (95% CI [0.053–1.32], Stanirowski et al, 2016b) to 0.900 (95% CI [0.341–2.38], Bua et al, 2017).
Cochran’s χ2 test for heterogeneity revealed no evidence for statistical heterogeneity (χ2(4)=2.80; p=0.592). I2 was 0.0%, indicating very low levels of heterogeneity and implying that the results of fixed and random effects models would be similar.
τ2 (effect size variance) was zero, implying a lack of substantial variability in the true effect sizes due to consistent methodologies or similar contexts. The zero value corresponds to a 95% predictive interval for the effect (odds ratio) of [0.40–0.87]. The implication of the zero τ2 value is to narrow the prediction interval. The data is summarised in a forest plot [Figure 3].
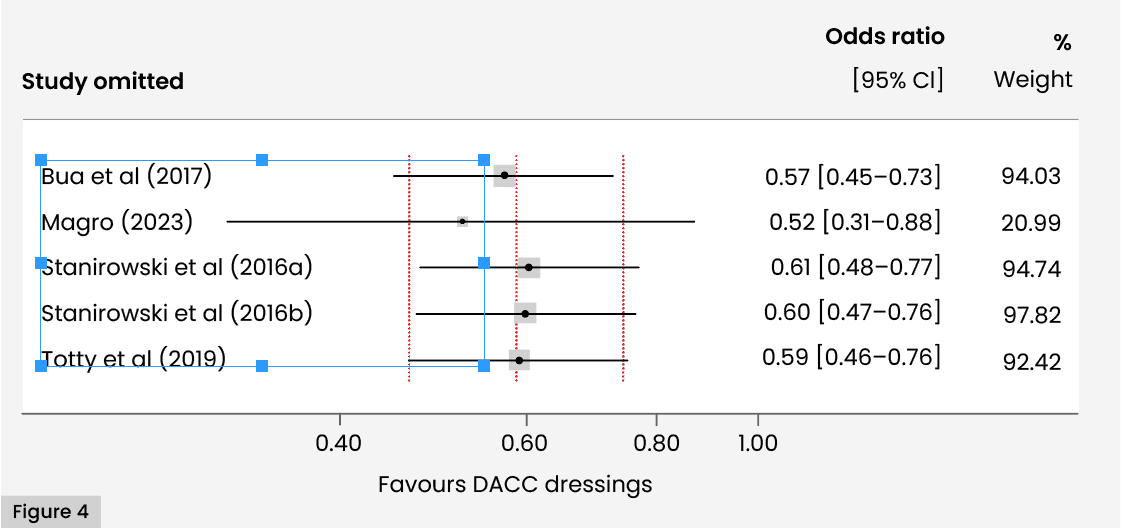
Findings were dominated by the results of Magro (2023). However, an influence plot revealed that no study, including the study of Magro, was excessively influencing the synthesised estimate, with the estimates from all the omitted meta-analyses lying within acceptable limits (95% CIs associated with the estimate of the combined analysis) [Figure 4].
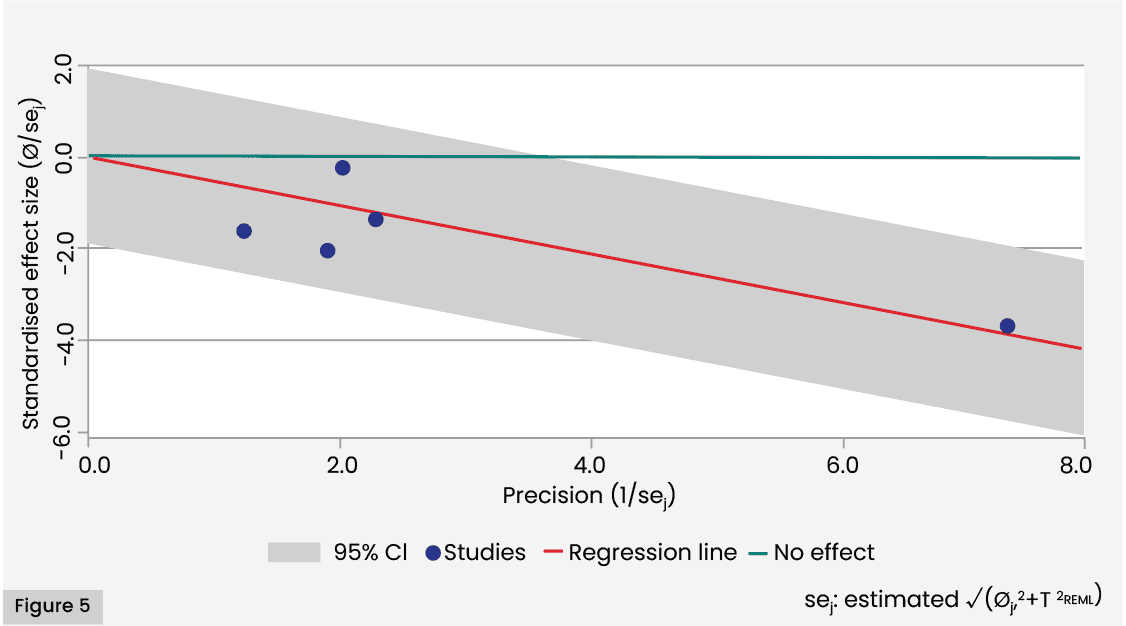
A Galbraith plot for the SSI outcome revealed that no included studies were outside the 95% CI region (given by the shaded area of the plot). The figure also illustrates diversity of levels of precision of effects, with points corresponding to most studies being clustered close to the origin, and a single point (corresponding to Magro, 2023) some distance from the origin [Figure 5].
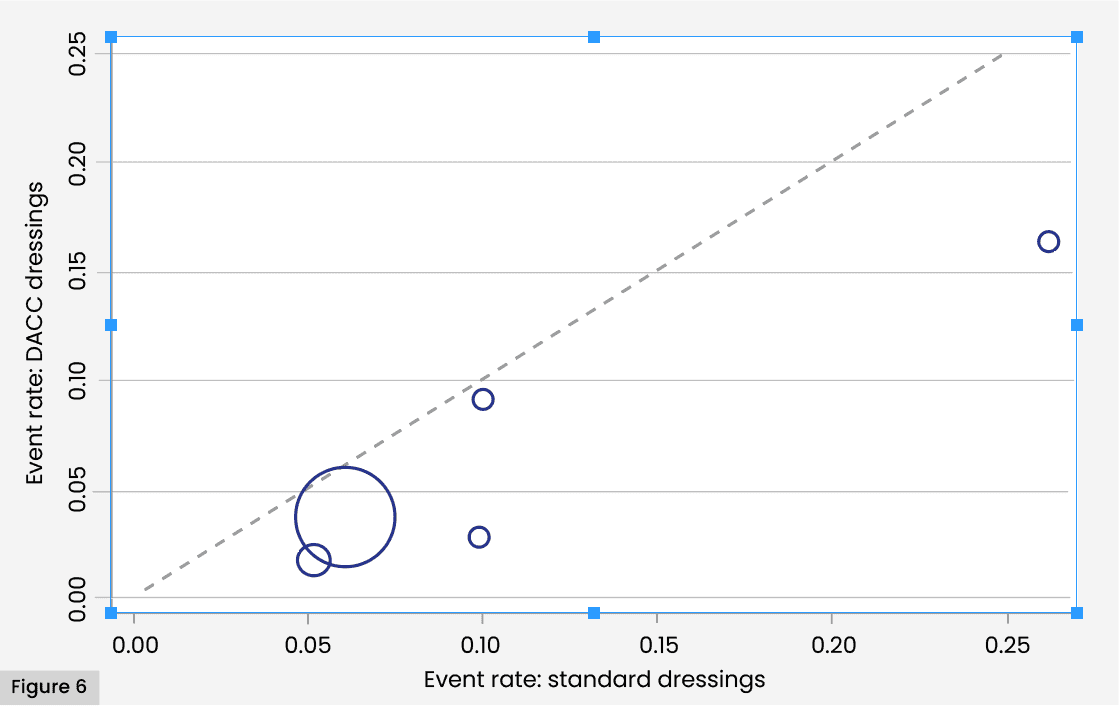
The L’Abbé plot illustrates the uniformly better performance of the DACC dressing treatment groups across studies. The higher magnitude of rates in both groups found by Totty et al (2019) represented by the isolated point at the right-hand end of the graph, is apparent. Substantive difference in event rates are also apparent in both this study and that of Stanirowski et al (2016a). However, no clear interaction between baseline risk and treatment efficacy is clear from the plot [Figure 6].
Assessment of evidence quality
The evidence in favour of DACC-coated dressings was judged to be high, according to the Cochrane-based criteria adopted to assess quality of evidence. Baseline quality of the included studies was high, with three randomised controlled trials and two quasi-experimental studies.
The risk-of-bias assessment judges all included papers to be at low or medium risk of bias. High levels of consistency were observed across studies, as evidenced by low variance of point estimates across studies and a substantial overlap of CIs, indicating that no evidence that variation is more than what one would expect by chance alone. Statistical testing confirmed these findings, with no evidence for statistical heterogeneity according to Cochran’s Q test of the null hypothesis that all studies have the same underlying magnitude of effect; and a negligible value of the I2 statistic, which quantifies the proportion of the variation in point estimates due to among-study differences. Precision of evidence was also demonstrated by the 95% CI [0.46–0.75] for the odds ratio, which did not cross the clinical decision threshold of a 20% reduction in odds of SSI. The evidence for publication bias is unclear, due to the small number of included studies. Hence, there was no evidence to downgrade evidence quality from baseline assessments on the grounds of inconsistency, indirectness or imprecision.
Discussion
This systematic review with meta-analysis explored the clinical effectiveness of DACC-coated wound dressings in reducing risk of SSI. We focused on SSI prevention within 30 days of procedure for this review. We excluded studies which were not comparing DACC-coated dressings with a suitable control or those which only reported outcomes beyond the scope of this analysis (e.g. pain).
DACC-coated dressings lead to lower rates of SSI within 30 days – treating patients with DACC-coated dressings almost halves the risk of SSI within this timeframe [Figure 3]. However, this conclusion is based on studies concerned with C-sections and vascular surgery only. There were no included studies based on other types of surgical procedures.
One quasi-experimental study, Magro (2023) utilised a much larger sample size than the other studies. However, this study reported very similar outcomes to the effects recorded in most of the other studies. Hence, despite the imbalance in sample sizes created by the inclusion of this study, there is no evidence that this study is exerting undue influence on the findings, according to the sensitivity analysis. Notwithstanding this, the synthesised estimate and associated CI reported in the meta-analysis are very similar to those obtained by Magro (2023) alone; reflecting the high weighting of this study in the meta-analysis.
The two non-randomised studies included in the meta-analysis are considered to provide levels of evidence approaching that of RCTs: the very short timeframe between data collection periods minimises the risk of systematic population differences, clinical practices or external factors. There is no evidence that the use of historical controls has led to non-comparable groups; with corresponding risks of selection bias or confounding.
SSI incidence within 30 days in patients treated with DACC-coated dressings were universally lower than SSI incidence within 30 days in patients treated under standard care, as illustrated in the L’Abbé plot; which also suggests that Totty et al (2019) can be considered to be an outlier in terms of SSI rates in both groups. The plot also reveals that Stanirowski (2016a) shows the greatest disparity in SSI rates across study groups. However, the Galbraith plot confirms that the variation of effects over all studies is within expected limits. The low levels of statistical heterogeneity revealed by the meta-analysis is likely to reflect correspondingly low clinical and methodological heterogeneity within the studies.
We found the quality of evidence as measured by risk of bias, indirectness, imprecision and inconsistency to be high.
DACC-coated dressings offer an innovative approach to surgical wound care by using a purely physical method to prevent microbial colonisation and reduce the risk of SSI. The hydrophobic surface of DACC dressings minimises the use of antimicrobials and the emergence of resistance (Karanika et al, 2016). This is especially important considering growing concerns concerning antimicrobial resistance.
Prevention of SSI is essential because delayed healing of surgical wounds costs the NHS an estimated £957.4 million–£985.8 million annually (Guest et al, 2017). The UK Health Security Agency (2022) estimates that SSIs occur in 0.4–11.3% of cases.
Limitations
Only PubMed and Scopus were searched. We were unable to assess the evidence for possible publication bias due to low numbers of included studies. All the included studies were concerned with SSI following C-section or vascular surgery; hence, the extent of surgical procedures included is limited. It is recommended future research undertaken should explore a range of procedures.
Conclusion
This systematic review and meta-analysis highlighted the effectiveness of DACC-coated wound dressings in reducing the risk of surgical site infections (SSI) within 30 days following a procedure. The findings indicate that these dressings can nearly halve the incidence of SSI during this period. Further investigation is required to determine the broader relevance of these results across various surgical contexts.
References:
