Wound care product-induced dermatitis: a critical narrative review on excipients, preservatives and skin biocompatibility
Received:
Accepted:
Published:
Authors: Leticia Vallejo-Carmona, Oscar Caicho-Caicedo, Enrique Chavez-Cifuentes
Citation:
Vallejo-Carmona L, Caicho-Caicedo O. Chavez-Cifuentes E (2025) Wound care product-induced dermatitis: a critical narrative review on excipients, preservatives and skin biocompatibility. Global Wound Care Journal 1(2): 62–71
Conflicts of interest:
LV-C is on the Global Wound Care Journal editorial board; this did not influence peer review
Corresponding author
Leticia Vallejo-Carmona, Ave. Domenech 385, San Juan, Puerto Rico 00918. Email: vallejol1@uagm.edu
DOI: https://doi.org/10.63896/gwcj.1.2.62
Periwound skin is a vulnerable structure constantly exposed to adhesives, dressings, cleansing agents and other topical wound care products containing excipients and preservatives with sensitising potential. Despite their widespread use in clinical practice, multiple studies have established a strong association between these components and cutaneous adverse reactions, particularly irritant and allergic contact dermatitis (Johansen et al, 2015; Alavi et al, 2016, 2020; Thornton et al, 2021). These reactions are frequently underdiagnosed, potentially leading to delayed healing, increased risk of secondary infections and a diminished patient experience. This critical narrative review synthesises current evidence on the prevalence, pathophysiology and risk factors associated with wound care product-induced dermatitis. Key sensitising agents, such as methylisothiazolinone, benzyl alcohol and colophony, are identified. The review emphasises the critical role of dermatologists in differential diagnosis using patch testing, alongside the proactive role of wound care nurses in early detection, documentation and prevention. Finally, safe, hypoallergenic and biocompatible alternatives are proposed to support an interdisciplinary approach that prioritises periwound skin integrity as a cornerstone of wound healing strategies.
Advanced wound care has evolved significantly over the past few decades, with a growing arsenal of dressings, topical solutions, protective films, and adjuvant technologies. However, the design of these products frequently prioritises therapeutic function over dermatological tolerance, leaving the impact of their excipients and preservatives on the perilesional skin, a particularly vulnerable area, in the background (Augustin et al, 2021).
Contact dermatitis induced by medical devices represents a growing phenomenon in clinical practice, with reports estimating a prevalence of up to 25% in patients with chronic wounds exposed to adhesives and dressings for prolonged periods (Khalid et al, 2022). These reactions are often confused with local infections, wet dermatitis, or nonspecific eczema, leading to significant clinical underdiagnosis and misprescribing of antimicrobials or antifungals (Kleinnijenhuis et al, 2020).
In this scenario, collaboration between dermatologists and nurses specialised in wounds becomes essential. The dermatologist provides diagnostic tools, such as the patch test, and a deep understanding of skin immune reactivity, while the nurse offers practical knowledge on the continuous application of products and clinical monitoring of the skin (Imbesi et al, 2011; Beeckman et al, 2020).
This critical narrative review seeks to systematise the available knowledge on the mechanisms of skin irritation and sensitisation induced by common products in wound care. At the same time, it proposes criteria for a safer selection of inputs, promoting clinical practices based on biocompatibility, prevention of iatrogenic damage and respect for skin integrity.
Pathophysiology of contact dermatitis on perilesional skin
Contact dermatitis is an inflammatory manifestation of the skin that can be classified into two main forms: irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD), both relevant in the context of wound care, especially in perilesional areas subjected to multiple adhesive and topical products (Rangaraj et al, 2014).
Irritant contact dermatitis
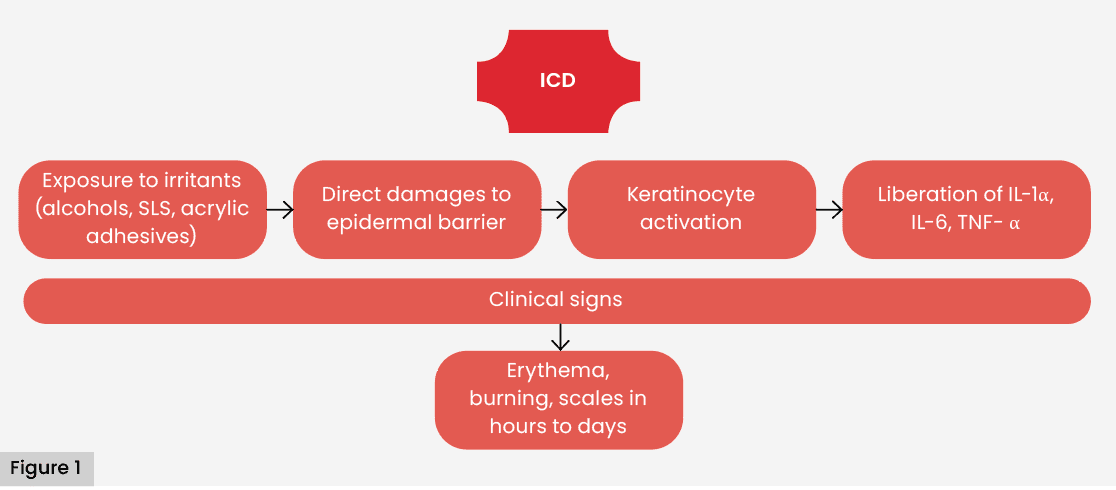
ICD represents most of the contact dermatitis in device/dressing related cohorts, commonly reported as around 60–80%, although proportions vary by setting and methodology (e.g. specialty clinics versus general dermatology). Therefore, we avoid implying a global fixed rate. Its pathophysiology is based on the direct disruption of the epidermal barrier and the activation of keratinocytes by irritants, such as alcohols, surfactants or acrylic adhesives, which triggers an inflammatory cascade mediated by proinflammatory cytokines, such as IL-1α, IL-6 and TNF-α (Proksch et al, 2008; Figure 1). This type of reaction does not require prior sensitisation and can develop rapidly after repeated or prolonged exposure, particularly in fragile or macerated skin.
In patients with exudative wounds or under occlusion, periwound skin shows increased humidity, pH shifts and barrier impairment—changes that collectively heighten permeability to irritants (Patterson et al, 2021). Scratching, mechanical friction of the dressing and prolonged occlusion contribute to aggravating epidermal damage.
Allergic contact dermatitis
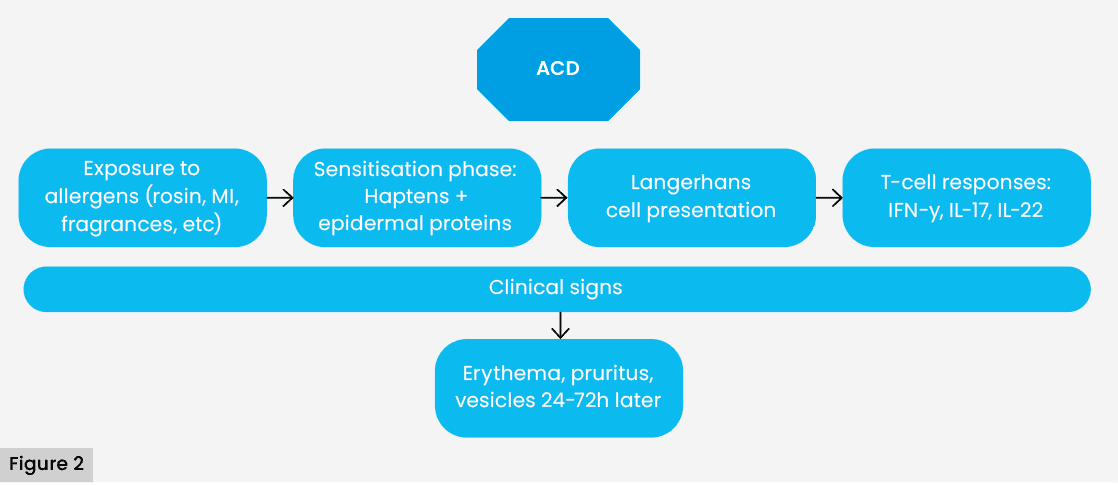
Unlike ICD, ACD is a type IV delayed hypersensitivity reaction, mediated by T lymphocytes, that requires an initial sensitisation phase. During this phase, low molecular weight haptenes, such as rosin, methylisothiazolinone (MI) or Peru balsam, combine with epidermal proteins, forming antigenic complexes that are taken up by Langerhans cells. These cells migrate to lymph nodes, where they induce the differentiation of specific T lymphocytes (Basketter et al, 2015).
In subsequent exposures, these activated T lymphocytes return to the contact site, release inflammatory cytokines (IFN-α, IL-17, IL-22) and cause the appearance of erythema, vesiculation, pruritus and desquamation, usually around application of the product (Uter et al, 2018). Reactions may occur 24–72 hours after contact and persist for several days even after removal of the causative agent [Figure 2].
Favourable conditions in chronic wounds
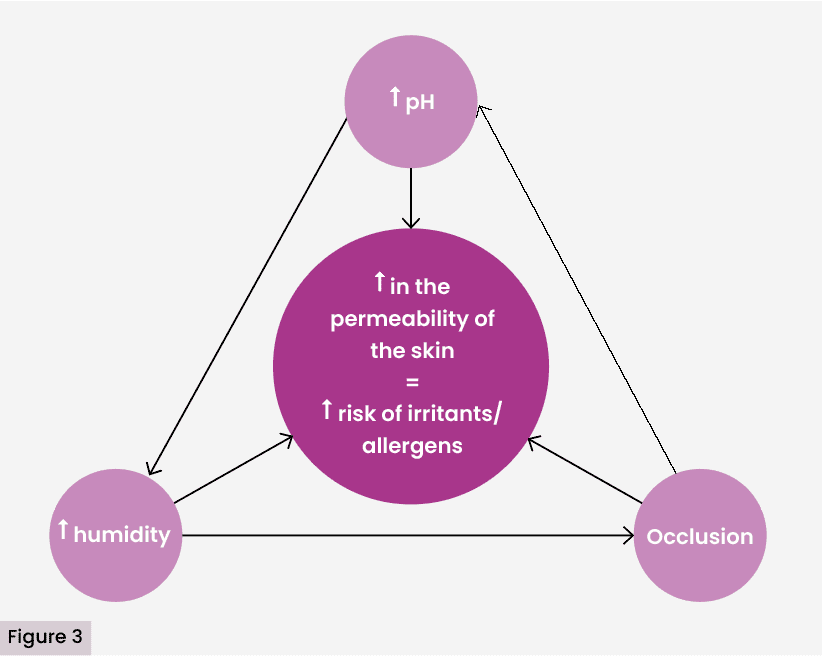
Recent studies have shown that the microenvironment of the chronic wound presents an imbalance between oxidative stress and antioxidant capacity, which contributes not only to the deterioration of the healing process, but also to a greater vulnerability of the perilesional skin to external chemical aggressions [Figure 3] (Vallejo-Carmona et al, 2024). The alteration of extracellular pH, added to the excess of reactive oxygen species (ROS), generates conditions that compromise the integrity of keratinocytes and fibroblasts, facilitating the entry of haptens and sensitising excipients.
Patients with chronic wounds are particularly exposed to contact dermatitis due to several factors:
- Prolonged use of products with multiple chemical components (Löffler and Effendy, 2020).
- Loss of barrier function due to chronic inflammation or maceration (Augustin et al, 2021).
- Repeated exposure to the same chemical product or class (Khalid et al, 2022).
- Immunological comorbidities or diabetes mellitus, which alter the cutaneous inflammatory response (Korting et al, 2005).
Up to 15–35% of patients with chronic ulcers have been reported to develop sensitisation to one or more components of topical products used during treatment (Thornton et al, 2021).
Additionally, in patients with chronic diseases such as diabetes, it has been documented that the wound microenvironment presents a significant biochemical imbalance, characterised by a sustained increase in oxidative stress and alterations in extracellular pH (Vallejo-Carmona et al, 2024). These factors directly contribute to keratinocyte, fibroblast and endothelial cell dysfunction, hindering their migration, proliferation and reparative capacity.
The accumulation of reactive oxygen species (ROS) results in prolonged inflammation and degradation of the extracellular matrix, conditions that further weaken the perilesional skin barrier and increase its permeability against irritants and sensitising haptens. In turn, alkalinisation of wound pH favours the activity of dermal proteases, such as matrix metalloproteinases (MMPs), exacerbates cell dysfunction, and creates a more favourable environment for microbial colonisation, thus amplifying the risk of contact dermatitis in these vulnerable areas (Vallejo-Carmona et al, 2024).
Products commonly used in wounds and their irritating or sensitising potential
The therapeutic approach to wounds involves the frequent application of products designed to protect, hydrate, debride or isolate the injury. However, multiple studies have documented that several of these products contain substances with irritant or allergenic potential that, when applied repeatedly to compromised skin, can trigger contact dermatitis (Lachapelle et al, 2019; Thornton et al, 2021).
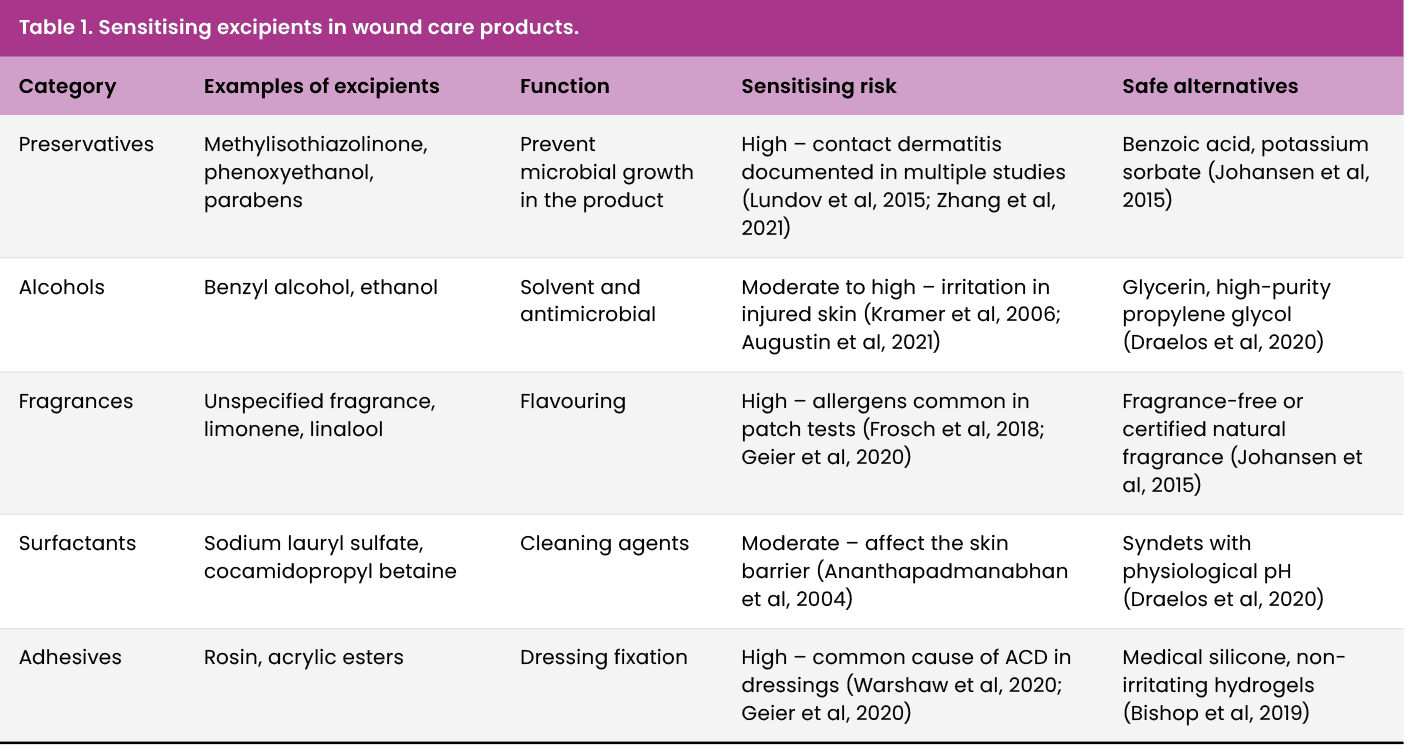
In this review, the therapeutic approach to wounds refers to four routine intents applied to the periwound: protect (e.g. barrier films, bordered dressings), hydrate (emollients/humectants to restore the acid mantle), debride (enzymatic or autolytic agents that may contact periwound during application) and isolate (zinc oxide or ostomy type pastes/powders that prevent maceration). Each intent carries distinct dermatitis risks driven by excipients (preservatives, fragrances, adhesives and surfactants); safer biocompatible alternatives are summarised in Table 1.
Adhesive dressings and barrier films
Acrylic adhesives and rosin derivatives are widely used to secure dressings and medical devices. These compounds can cause both irritant dermatitis and type IV allergic reactions, especially in patients with fragile skin or exposed to frequent dressing changes (DeKoven et al, 2018). Alcohol-based protective films also pose a risk in macerated skin, as they promote the disruption of the epidermal barrier (Beeckman et al, 2020).
Several studies recommend the use of medical silicone adhesives, which show lower sensitising capacity and good skin tolerance, even in prolonged applications (Bishop et al, 2003).
Creams, pastes and emollients
Many topical products applied to perilesional skin contain lanolin, an emollient derived from sheep’s wool, the unsaponifiable fraction of which has been associated with allergic reactions in up to 12% of sensitised patients (Goossens et al, 2020). Likewise, the use of MI as a preservative in creams has been strongly questioned due to its high sensitising capacity, which has led to its prohibition in cosmetic leave-in products in the European Union (Lundov et al, 2009).
It is recommended to select dermatologically tested products, free of isothiazolinones, fragrances and other non-essential preservatives, prioritising biocompatible alternatives (Johansen et al, 2015).
Cleaning solutions and soaps
Hospital-grade cleansers often contain anionic surfactants, such as sodium lauryl sulfate (SLS), which have been shown to cause irritation in intact skin and significant disruption of the stratum corneum (Ananthapadmanabhan et al, 2004). In wounds, the risk is increased by the previous alteration of the barrier function.
Alternatively, syndets (synthetic detergents) with physiological pH, without SLS or fragrances, have shown a better tolerance profile and adequate cleaning efficacy without risk of sensitisation (Draelos et al, 2020).
Antiseptics and topical solutions
Antiseptics, such as povidone-iodine and benzyl alcohol, are effective in reducing microbial load, but they can cause irritation, especially when applied to large areas, macerated skin, or skin with epidermal loss (Kramer et al, 2006). In addition, in patients with an allergic predisposition, they can trigger delayed hypersensitivity reactions.
Recent studies suggest the controlled use of non-cytotoxic antiseptics such as polyhexanide or chlorhexidine, always considering the balance between antimicrobial efficacy and preservation of skin integrity (Leaper et al, 2012).
Antiseptic stewardship in wound care: Use non cytotoxic agents only when indicated (bioburden at risk; peri-procedural prophylaxis), for the shortest effective duration and avoid routine, prolonged use on intact periwound. Prefer polyhexanide (PHMB) or balanced chlorhexidine where appropriate; avoid alcohol based films on macerated skin. Monitor for irritation on serial assessments and de-escalate once control is achieved (Kramer, 2006; Leaper, 2012).
Role of the dermatologist in the evaluation of dermatitis in wound care
In advanced wound care, early and accurate diagnosis of topical-induced dermatitis requires specialised clinical evaluation. The dermatologist, as an expert in the pathophysiology of skin reactions, plays a decisive role in the identification of lesions of allergic or irritative origin, differentiation with other dermatoses and personalised therapeutic guidance.
Clinical evaluation and differential diagnosis
Manifestations of a contact dermatitis can mimic signs of superficial infection, wet eczema, peristomal dermatitis, or even wound bed complications, leading to a high risk of diagnostic error if a systematic dermatological approach is not applied (Kleinnijenhuis et al, 2020).
The dermatologist should assess:
- Distribution and pattern of lesions: ACD tends to respect non-exposed areas, while ICD tends to have diffuse and longer borders (Uter et al, 2018).
- Chronology regarding exposure to the product: ICD can appear rapidly, while ACD typically manifests 24–72 hours after exposure (Brasch et al, 2014).
- Association with multiple products: polyexposed patients (adhesives, dressings, films, cleaners) are at increased risk of cross-sensitisation or multiple sensitisation (Goossens et al, 2020).
Correct differentiation allows avoiding unnecessary treatments (such as topical or systemic antibiotics) and suspending the causative agent.
Use of patch tests
The diagnostic standard to confirm ACD is the patch test, a widely validated tool in occupational and clinical dermatology. This technique consists of applying standardised allergens to the patient’s back, including those present in dressings, creams or used solutions, and assessing the skin response after 48 and 72 hours (Johansen et al, 2015).
Studies have shown that up to 20% of patients with chronic venous ulcers have positive reactivity to products used in their usual treatment, the most frequent being rosin, Peru balsam, neomycin and MI (DeKoven et al, 2018; Lachapelle et al, 2019).
The test also allows the identification of hidden or undeclared allergens on the labelling of medical products, a situation frequently reported in industrial dressings (Basketter et al, 2015).
Interdisciplinary contribution in prevention and education
The dermatologist, in conjunction with wound nurses, may:
- Provide advice for the selection of safe products according to risk profile.
- Establish protocols for rotation or reduction of exposure to known excipients.
- Develop educational guides for healthcare workers on early identification of product-based dermatitis (Beeckman et al, 2020).
Evidence suggests that the implementation of interdisciplinary wound units that include dermatology significantly improves clinical outcomes in patients with dermatitis secondary to topical treatment (Augustin et al, 2021).
Clinical recommendations from specialised nursing
Specialised wound nursing plays a fundamental role in the prevention, identification and management of product-induced dermatitis. As a professional who directly applies and monitors dressings, adhesives, topical solutions and barrier films, nurses are in a unique position to recognise early signs of skin reactivity and prevent further complications. This proactive approach aligns with evidence-based clinical practice and with international patient safety standards (Beeckman et al, 2020).
Systematic assessment of perilesional skin
It is recommended to implement a routine and documented assessment of the perilesional skin, including parameters such as coloration, texture, presence of erythema, scaling, maceration, pruritus or localised pain [Table 2]. Tools such as the Bates-Jensen skin condition scale or adapted perilesional integrity scoring systems make it possible to objectify changes and make timely clinical decisions (Baranoski and Ayello, 2021).
Red, irritated or frizzy skin with repeated use of the same product should be considered a warning sign and require therapeutic reevaluation.
As a practical tool for clinical decision-making, a fast-acting algorithm that guides health personnel in the identification, documentation, and early management of dermatitis induced by wound care products is presented below.
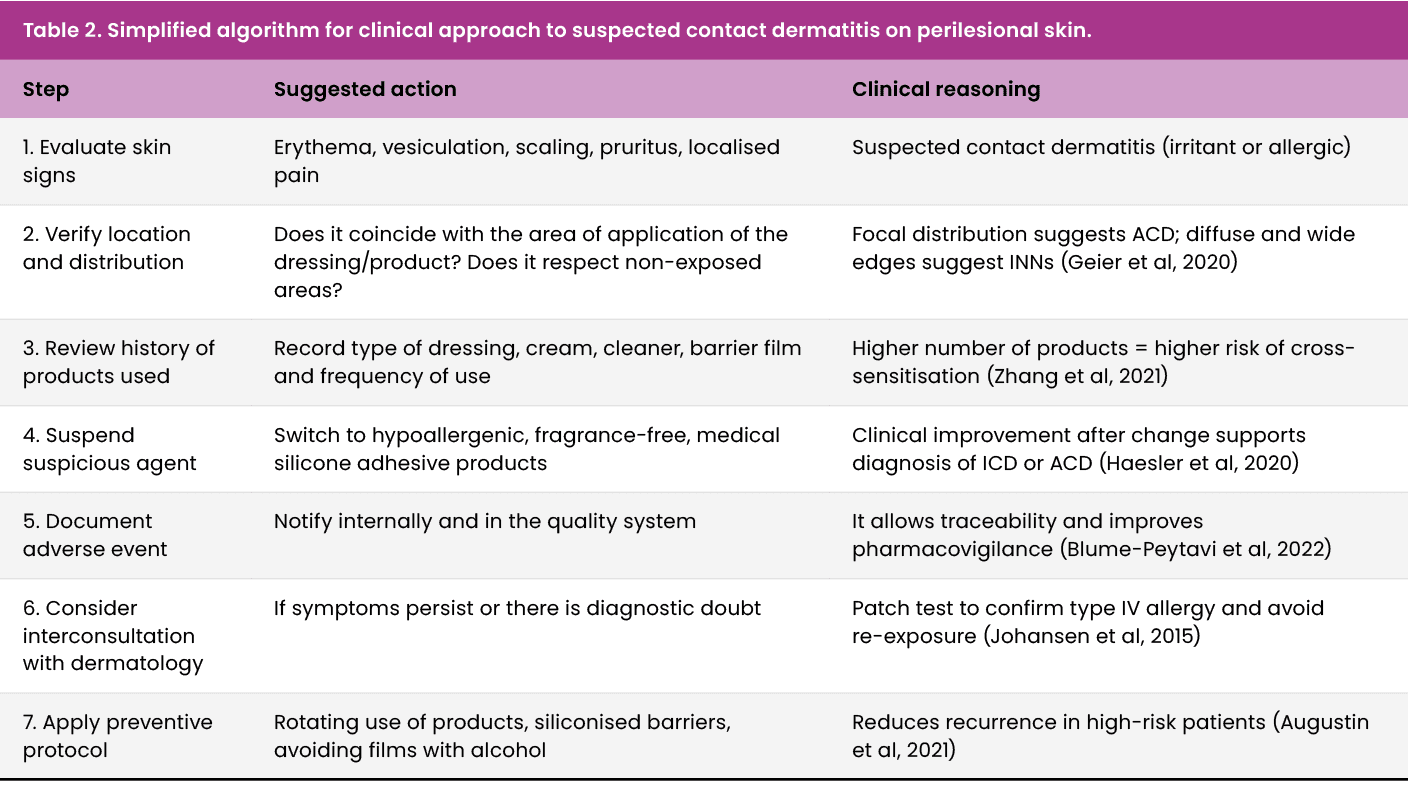
Documentation and reporting of skin adverse events
Underreporting of device-related skin reactions is well documented; therefore, reporting is an interdisciplinary responsibility. All healthcare professionals —including nurses, dermatologists, prescribing clinicians and pharmacists — should:
Record the event in internal quality and patient safety systems.
Document brand, lot number and product type.
Notify the wound team to consider safe substitutions.
Nurse-led early detection, dermatologist confirmation through patch testing when indicated, prescriber review, and pharmacist checks collectively improve traceability and strengthen post-market surveillance for non-pharmacological medical products.
Criteria for the choice of biocompatible products
Evidence supports the selection of dermatologically tested inputs, fragrance-free, high-risk preservative-free, and with medical silicone adhesives, especially in patients with aged, macerated skin, or with a history of allergies (DeKoven et al, 2018).
It is recommended to avoid the use of:
- Alcohol-based barrier films on macerated or eroded perilesional skin.
- Creams with lanolin or MI in sensitised patients.
- Adhesives with rosin or acrylic esters in friction areas.
Safe alternatives include resin-free hydrocolloid-edged dressings, soft silicones, syndet pH-neutral cleaners and volatile solvent-free barriers (Lundov et al, 2009; Figure 4).
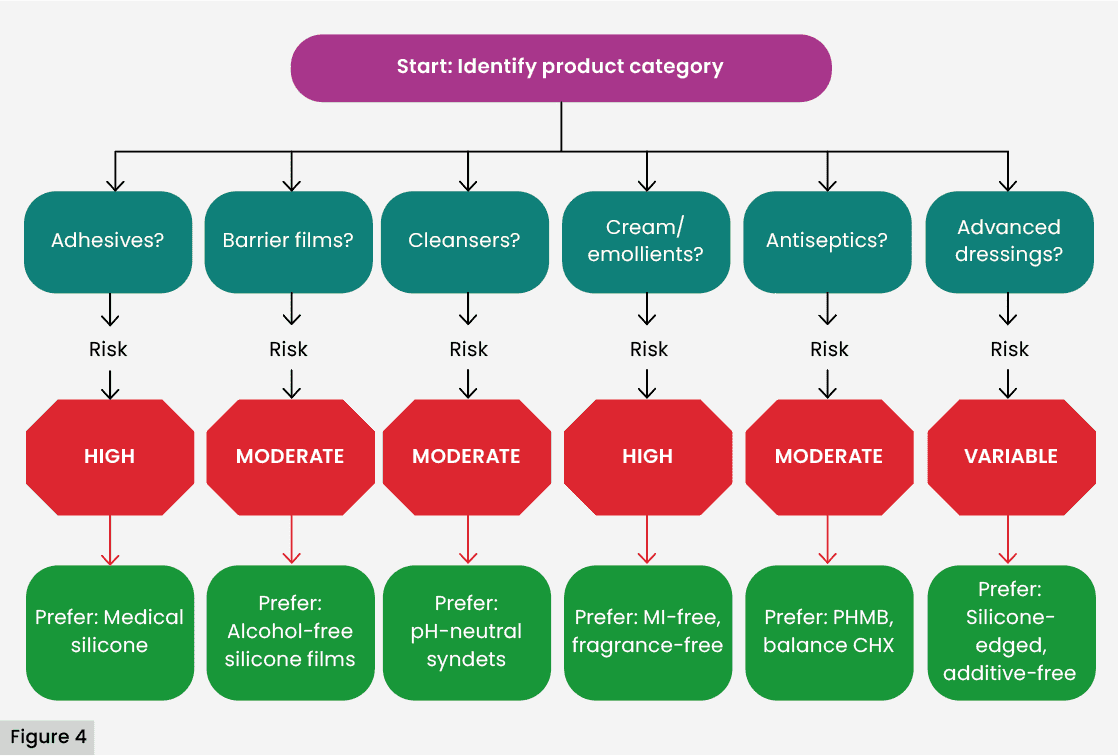
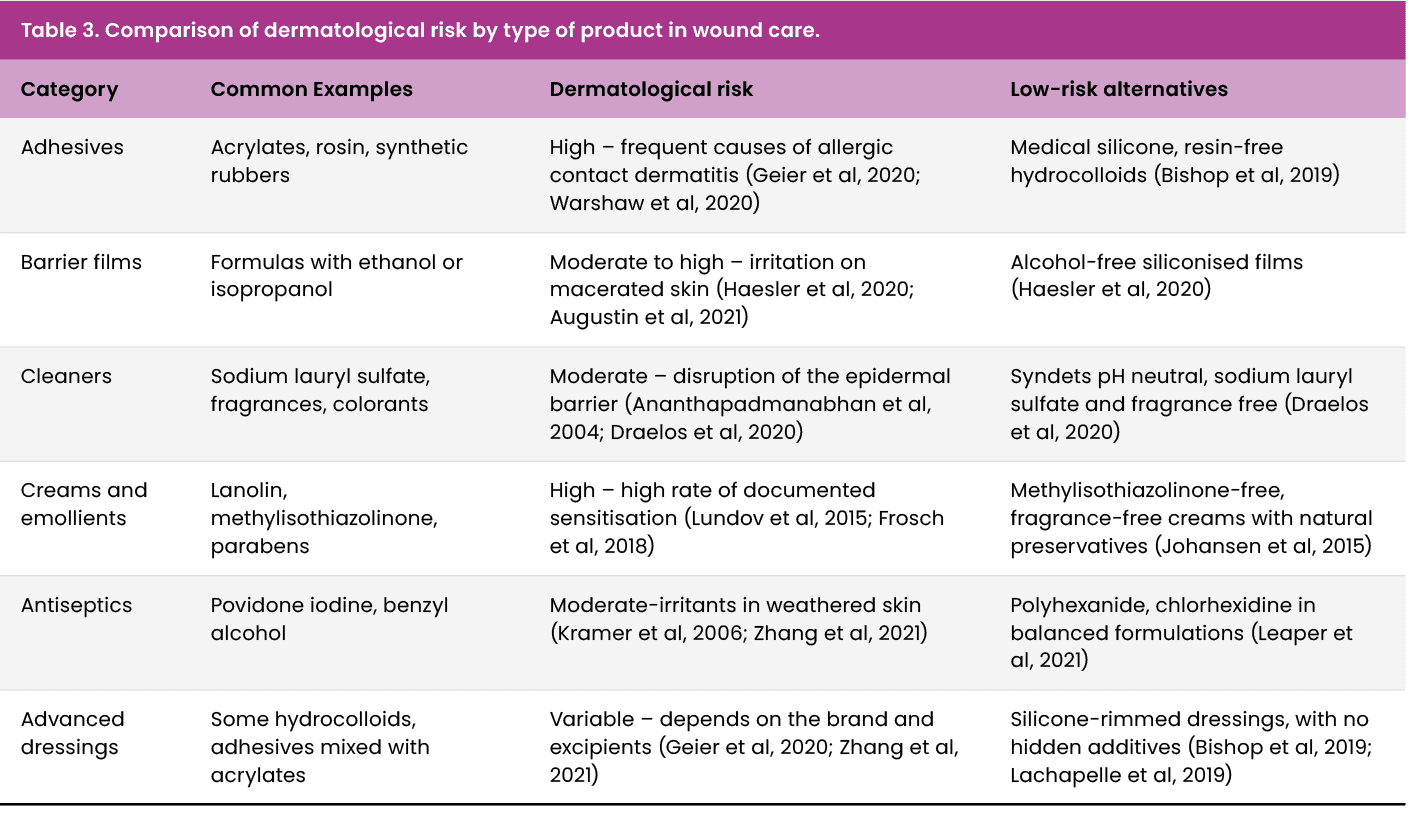
Table 3 summarises the main groups of products used in wound care, categorising them according to their dermatological risk reported in the literature. This tool seeks to support safer clinical selection, especially in patients with fragile, macerated skin or with an allergic history.
Continuing education and prevention protocols
The inclusion of content on contact dermatitis in continuing wound education programmes is essential. Studies have shown that clinical training in early identification of dermatological lesions improves adherence to treatment and reduces associated complications (van Montfrans et al, 2014).
In addition, the implementation of product rotation protocols, “adhesive rest” in critical areas, and preventive use of non-irritating siliconised barrier films is recommended, especially in patients with multiple skin comorbidities.
Innovations and proposals for safe materials
The development of medical technologies applied to wound care has given rise to a new generation of products designed not only to optimise healing, but also to minimise the impact on perilesional skin, especially in patients with skin fragility or a history of contact dermatitis. These innovations seek to reduce the content of aggressive excipients, improve dermatological tolerance and increase the biocompatibility of materials (Kirsner et al, 2020).
Medical Silicone Adhesives and Soft Fixing Technologies
Traditional adhesives based on acrylates, rosin and synthetic rubbers exhibit a considerable rate of irritation and sensitisation, especially in repeated applications (DeKoven et al, 2018). In contrast, therapeutic-grade medical silicone adhesives have positioned themselves as a safe alternative, thanks to their low antigenic profile, their ability to maintain adhesion without damaging the stratum corneum and their easy atraumatic removal (Bishop et al, 2003).
In addition, dressings with soft silicone edges have been shown to significantly reduce the risk of skin tears and pain when removing, particularly benefiting older adults and patients with atrophic skin (Larouche et al, 2018).
Products without isothiazolinones or fragrances
In response to the accumulating evidence on the high sensitising capacity of MI and its derivatives, numerous manufacturers have begun to formulate topical products without this preservative, replacing it with alternatives such as phenoxyethanol or validated natural preservatives (Lundov et al, 2009).
At the same time, the elimination of artificial fragrances, recognised as one of the main triggers of allergic dermatitis, has been promoted, even in products labelled as “hypoallergenic” (Johansen et al, 2015).
Alcohol-free barrier films and biocompatible polymers
The new generations of protective films use high molecular weight acrylic copolymers, volatile silicones and non-irritating film-forming agents, avoiding the use of ethanol or isopropanol as a vehicle (Beeckman et al, 2020). These products have been shown to be effective as physical barriers without disrupting the skin’s lipid barrier or visible inflammatory reactions (Augustin et al, 2021).
In patients with compromised skin, the use of these films has shown a reduction in the incidence of contact dermatitis by up to 60% compared to traditional formulations (Thornton et al, 2021).
Technological advances: smart dressings and skin sensors
The most recent innovations include smart dressings, capable of monitoring wound bed moisture, pH, temperature and bacterial load in real-time, without the need for removal (Mostafalu et al, 2018). By avoiding frequent changes and unnecessary handling, these devices significantly reduce skin exposure to irritants.
In addition, smart bioadhesives are being developed, formulated with synthetic or natural polymers that dynamically adapt to the skin without inducing inflammation or altering the skin microbiota (Yang et al, 2022).
Discussion
Dermatitis induced by wound care products is an underestimated clinical phenomenon, but with significant therapeutic implications. Throughout this review, it has been shown that multiple routinely used inputs (such as adhesive dressings, protective films, cleaning solutions and emollients) contain excipients and preservatives with irritating or sensitising potential that compromise the integrity of the perilesional skin (Uter et al, 2018; Thornton et al, 2021).
One of the most relevant findings is the high prevalence of irritant contact dermatitis (ICD), especially associated with the repeated use of films with alcohol and anionic surfactants, such as sodium lauryl sulfate (Ananthapadmanabhan et al, 2004). The perilesional skin, already altered by exudate, maceration or friction, has a diminished barrier function, which facilitates the penetration of irritating substances (Proksch et al, 2008). This clinical condition, in many cases, is indistinguishable from an infection or wet eczema, which leads to therapeutic errors, such as the unnecessary application of antimicrobials (Kleinnijenhuis et al, 2020).
In complex clinical contexts, such as chronic wounds, increased wound pH and sustained oxidative stress have been linked to cellular dysfunction, decreased angiogenesis, persistent inflammation and increased susceptibility to infections or exacerbated inflammatory reactions, including contact dermatitis (Vallejo Carmona et al, 2024).
ACD, although less common, represents an even greater diagnostic challenge. Prolonged exposure to common allergens such as rosin, lanolin, fragrances or MI can trigger type IV hypersensitivity reactions, especially in polyexposed patients (Basketter et al, 2015; Lachapelle et al, 2019). The use of the patch test as a diagnostic tool has proven to be essential to confirm the allergic aetiology and guide the elimination of the causative agent (Johansen et al, 2015).
From a transdisciplinary perspective, this problem requires close collaboration between dermatology and specialised nursing. While the dermatologist provides the diagnostic tools and immunological knowledge, the nurse is the first to observe changes in the skin, identify incipient signs of damage and suggest preventive interventions (van Montfrans et al, 2014; Beeckman et al, 2020).
As for therapeutic alternatives, technological advances offer promising solutions. Medical silicone adhesives, smart dressings and products without isothiazolinones and artificial fragrances have been shown to significantly decrease the incidence of adverse reactions, maintaining clinical efficacy (Bishop et al, 2003; Mostafalu et al, 2018). However, their availability and use remain limited in low-resource hospital settings, underscoring the need for institutional protocols that prioritise skin safety (Augustin et al, 2021).
Finally, this review raises an ethical and scientific alert: it is clinically questionable and ethically compromising to continue using products with high sensitising potential in the absence of clear regulation or an evaluation based on scientific evidence. The inclusion of clear warnings on labels, greater transparency about the complete composition of products and the implementation of post-market surveillance mechanisms are priority recommendations to move towards safer, more ethical and evidence-based care.
International regulations and gaps in the surveillance of excipients in wound products
Despite the growing recognition of skin reactions induced by medical products, international regulatory frameworks still have limitations in the requirement of detailed labelling and post-marketing control of excipients in dressings, solutions and barrier films.
The FDA (Food and Drug Administration) (FDA, 2020) and the EMA (European Medicines Agency) (EMA, 2021), classify many dressings and protective barriers as “class I or II medical devices”, which implies a lower degree of vigilance compared to pharmaceutical products. This classification means that components with sensitising potential — such as fragrances, MI or rosin — are not explicitly declared on the labelling, as they are not considered therapeutic active ingredients.
A recent study by Uter et al. (2018) showed that in more than 30% of the products analysed used in advanced wound care, allergenic excipients were not listed on the technical data sheets or labels, which makes diagnosis and prevention difficult. Illustrative clinical vignette: During a hospital quality audit, a patient was identified with recurrent periwound erythema associated with the use of an acrylic-bordered adhesive dressing. After switching to a medical silicone-bordered dressing, the reaction resolved within a few days. Patch testing confirmed allergy to colophony derivatives — an excipient not declared on the product’s label or technical datasheet. This finding highlights a significant limitation of current regulatory frameworks: the possibility that highly sensitising substances may be present but absent from declared composition, thereby hindering clinical diagnosis, prevention and traceability of adverse events. Such cases underscore the need for full transparency in the composition of medical devices and for more rigorous post-market surveillance mechanisms.
In Latin America, this situation is even more critical due to the absence of specific regulations regulating the non-therapeutic composition of dressings and their post-market surveillance. This regulatory vacuum generates unnecessary risk, especially in chronic or poly-exposed patients.
Therefore, it is urgent to promote regulatory frameworks that require the complete declaration of all components, including excipients and preservatives, as well as the implementation of mandatory reporting systems for cutaneous adverse events associated with the use of non-pharmacological medical products.
Regulatory vignette
In a hospital quality review, a patient with recurrent periwound erythema under an “acrylate-based” adhesive border dressing improved after switching to a silicone-bordered alternative. Subsequent patch testing revealed rosin (colophony) allergy; the original dressing’s technical sheet did not list rosin derivatives among excipients. This illustrates how Class I–II device labeling may omit sensitising excipients because they are not “active ingredients,” limiting clinician awareness and hampering post-market surveillance. Strengthening full composition disclosure and mandatory adverse event reporting for non pharmacologic medical products could address these gaps.
Conclusion
The presence of contact dermatitis induced by wound care products represents a common, underdiagnosed, and clinically significant complication. The scientific literature is clear in pointing out that excipients such as rosin, alcohols, iso-triazolinones, fragrances and certain adhesive polymers are agents with high sensitising or irritating potential, especially in skin already compromised by exudate, maceration or mechanical friction (DeKoven et al, 2018; Lachapelle et al, 2019; Thornton et al, 2021).
Despite this, the choice of clinical inputs continues to be guided, in many contexts, by commercial or availability criteria, which may limit the systematic integration of biocompatibility and dermatological safety criteria into clinical practice. This review proposes a paradigm shift: moving from the isolated efficacy of the product to a therapeutic strategy focused on the perilesional skin as an essential part of the healing process.
From clinical practice, this involves:
- Promote continuous education of health personnel in the identification of adverse skin reactions
- Promote pharmacovigilance of non-pharmacological medical products
- Implement interdisciplinary protocols that include the dermatologist as a key figure in the wound team
- Prioritise the use of hypoallergenic technologies, medical silicones and formulations free of sensitising components, especially in patients at high dermatological risk.
These proposals align with the highest level of evidence recommendations available internationally. Good practice guidelines published by Wounds International emphasise the need to maintain perilesional skin integrity through the choice of biocompatible products, ongoing skin assessment, and clinical education of staff (Haesler et al, 2020). Likewise, the updated TIME framework explicitly incorporates the perilesional skin condition as an essential component of the healing microenvironment, promoting preventive strategies against cutaneous adverse reactions (Leaper et al, 2012). In addition, the systematic review by Thornton et al. (2021) confirms that a significant proportion of topical products used in wound treatment contain allergens or irritants that are not always declared, reinforcing the urgency of adopting selection criteria based on dermatological safety and active surveillance.
The future of wound care must integrate principles of immunodermatology, materials science and patient-centred care. Only in this way will truly safe, ethical and evidence-based therapy be guaranteed.
